Q1) How can you purify your water when you are hiking? Name two or three possibilities. Compare these methods in terms of cost and effectiveness. Are any of these methods similar to those used to purify municipal water supplies? Explain.
There are many options to purify water while hiking.
– Boiling is the most certain way of killing all microorganisms. According to the Wilderness Medical Society, water temperatures above 160 degree F kill all pathogens within 30 minutes and above 185 degrees F, within a few minutes. So by the time it takes for the water to reach the boiling point from 160 degrees F, all pathogens will be killed, even at high altitude. To be extra safe, let the water boil rapidly for one minute, especially at higher altitudes since water boils at a lower temperature.
– Boiling however requires time and does not remove chemical contamination. It also requires fuel and may release soot and carbon dioxide to the atmosphere.

– Iodine is light sensitive and must always be stored in a dark bottle. It works best if the water is over 68 degrees F. Iodine has been shown to be more effect than chlorine-based treatments in inactivating Giardia cysts. However, some people may be allergic to iodine and cannot be used for water purification. Persons with thyroid problems or on lithum, women over fifty, and pregnant women should consult their physician prior to using iodine for purification. Also, some people who are allergic to shellfish are also allergic to iodine. If someone cannot use iodine, use either a chlorine-based product or a non-iodine-based filter.
– Iodine is easy and effective in around twenty minutes, but iodine should not be used long term. While iodine renders water bacteriologically safe, it does not remove chemical contaminations. Many people also dislike the taste of iodine-treated water.

– Filtration has the advantage that it does not require chemicals and removes many of the organisms not killed by boiling or iodine treatment.
– Filters are the most expensive option, a good filter pumps out good water in a few minutes and is reusable.

Municipal water preparation involves coagulation, sedimentation, filtration, and disinfection. Obviously more comprehensive, but like during hiking involves both filtration and disinfection – mainly chlorination.
Q2) Explain why desalination techniques, despite proven technological effectiveness, are not used more widely to produce potable drinking water.
Most common desalination techniques are reverse osmosis and distillation. However, both of these require a large amount of energy to salts from seawater or brackish water and thus are expensive methods. If a less expensive option is available such as hauling fresh water from a distance, then this option is used.

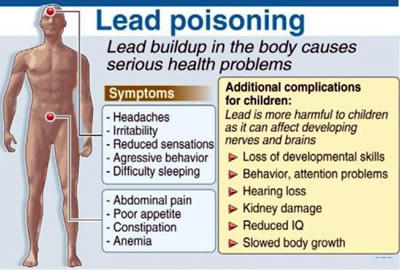
Q3) Water quality in a chemical engineering building on campus was continuously monitored because testing indicated water from drinking fountains in the building had dissolved lead levels above those established by NEA.
a) What is the likely major source of the lead in the drinking water?
b) Do the research activities carried out in this chemistry building account for the elevated lead levels found in the drinking water? Explain.
- The likely source of lead is from solder in the pipe joints or from lead pipes themselves.

- Research activities should not contribute to lead in the drinking water, assuming that any lead compounds are not dumped down the drain. Most chemistry experiments especially at the undergraduate level have been redesigned and do not involve lead compounds and other toxic metal ions. Recall that substances dumped into a sewage treatment system may end up downstream in someone else’s drinking water.
Q4) Some vitamins are water-soluble, whereas others are fat-soluble. Would you expect either or both to be polar compounds? Explain.
Only water-soluble vitamins would be expected to be polar molecules. Though a fat-soluble vitamin will often have individual polar bonds or small regions of the molecule, overall this is outweighed by nonpolar sections. Polar covalent bonds attract to water through hydrogen bonding and may allow the molecules to dissolve in water, while nonpolar covalent bonds favour interactions with the nonpolar chains in lipids.