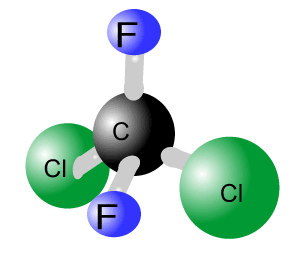
Question 1
(a)
The chemical forumla is C5H9N3.
(b)

(c)
N-H which is the ability to form hydrogen bonds with water.
Question 2
(a)
C16H21N3
(b)
The similarities are a two-carbon chain with a nitrogen at the end and a three-atom sequence of N-C-N, the spatial placement of these pieces, and the flat 5-ring of histamine can also be considered similar to the flat 6-ring of the antihistamine.
Question 3
| Drugs that produce a physiological response in the body |
|
| Drugs that inhibit the growth of substances that cause infections |
|
Question 4
(a)
Herbal supplement manufacturers provide labels on their products regarding ingredients used and whether it has complied with the relevant regulatory authorities’ standards.
Advice on dosage and usage compatibility are also provided within the package of the product itself.
(b)
Singapore has Traditional Chinese Medicine Practitioners Act (Chapter 333A), Health Products Act, Medicines Act, Poisons Act, and the Health Science Authority of Singapore (HSA) has issued guidelines regarding the use of traditional medicine materials.
Traditional Chinese Medicines are regulated by HSA and manufacturers must comply with a set of safety and quality criteria before thy are allowed to be sold.






 Today, we learnt about water. We didn’t know that there are so many methods of water purification and the impacts of each method. In Singapore, we have limited water resources, so we should all do our part to save water as there is a water hike recently announced by the Budget. Anyway, these are our group’s answers for this week’s questions:
Today, we learnt about water. We didn’t know that there are so many methods of water purification and the impacts of each method. In Singapore, we have limited water resources, so we should all do our part to save water as there is a water hike recently announced by the Budget. Anyway, these are our group’s answers for this week’s questions: ) bye!!!!!!!!
) bye!!!!!!!!
 ) Anyway, these are our group’s answers for this week’s questions:
) Anyway, these are our group’s answers for this week’s questions: