- Troposphere is the lowest level of the atmosphere, followed by the Thermosphere
- Good up High (Ozone is a protective gas in the Stratosphere)
- Bad Nearby (Pollutant in the Troposphere)
- Incomplete combustion results in the formation of Carbon Monoxide (CO)
- Catalytic converters catalyze the conversion of CO to C02 (reduces CO and also reduce VOC)
- Sulfur trioxide (SO3) forms corrosive sulfuric acid (H2S04) with water
- Sulfur dioxide reacts on the surface of a variety of airborne solid particles (aerosols), is soluble in water and can be oxidized within airborne water droplets, producing sulphuric acid.– (Ash hastens the formation of sulfur trioxide)
TED
AE 3
Question 1. From personal experience, state whether these processes are endothermic or exothermic. Give a reason for each.
- A charcoal briquette burns.
- Water evaporates from your skin.
- Ice melts.
Answer:
- Exothermic. Heat is released.
- Endothermic. Heat is absorbed to break bonds.
- Endothermic. Heat is absorbed to break the bonds.
Question 2. Chemical explosions are very exothermic reactions. Describe the relative bond strengths in the reactants and products that would make for a good explosion.
Answer: The relative bond strengths of the products are greater than that in the reactants, resulting in a very large exothermic reaction.
Question 3. How might you explain the difference between temperature and heat to a friend? Use some practical, everyday examples.
Answer:
- Heat is the total energy of the molecular motion in a substance.
- Temperature is the measure of the average energy of molecular motion of a substance.
- Heat is a type of energy but temperature is not energy.
- Heat cannot be directly measured with a device; temperature can be measured directly using devices such as thermometer.
For example, the hotness felt from a boiling kettle is the energy, and the degree of hotness can be measured using a thermometer.
Question 4. A premium gasoline available at most stations has an octane rating of 98. What does that tell you about:
- The knocking characteristics of this gasoline?
- Whether the fuel contains oxygenates?
Answer:
- It has a knocking characteristics of 98% isooctane and 2% heptane.
- No. Fuels that contains oxygenates would have octane rating over 100. Since the fuel has an octane rating of 98, it does not contain oxygenates.
AE 4
Question 1. Understanding Earth’s energy balance is essential to understanding the issue of global warming. For example, the solar energy striking Earth’s surface averages 168 watts per square meter (W/m2), but the energy leaving Earth’s surface averages 390 W/m2. Why isn’t Earth cooling rapidly?
Answer: The Earth does not cool rapidly because the atmosphere retains much of the emitted heat energy.
Question 2. Decide and explain whether the statement is correct or incorrect. Explain.
“This winter has lowered my concerns about global warming”
Answer: Incorrect. Global warming refers to an average increase in temperature of the Earth, and at the same time leads to erratic and more extreme weather conditions. Just because the a region of the Earth is experiencing a colder winter does not mean that the average global temperature has decreased.
Question 3. One of the first radar devices developed during World War II used microwave radiation of a specific wave range that triggers the rotation of water molecules. Why was the design not successful?
Answer: Absorption of microwave radiation by water in the atmosphere interferes with the detection of the intended objects.
Question 4. Now that you have studies air quality (Unit 1), stratospheric ozone depletion (Unit 2), and global warming (Unit 3), which do you believe poses the most serious problem for you in the short run (pick one and explain)? In the long run (Pick one and explain why)?
Answer:
- Short run: Air quality poses the most serious problem in the short run because it directly affects our health conditions and immediate environment in which we live in.
- Long run: Global warming poses the most serious problem in the long run because there is no direct and effective solution for it. Also, we can only control limited amounts of CO2 emitted but can never fully reduce the amount of CO2 reduced by humans.
AE 5
Question 1. How can you purify your water when you are hiking? Name two or three possibilities. Compare these methods in terms of cost and effectiveness. Are any of these methods similar to those used to purify municipal water supplies? Explain.
Answer:
- One method to purify water is to boil it. It disinfects the water by eliminating most microbes that can cause intestine-related diseases. Also, it does not affect the taste of the water. However, it cannot remove chemical toxins or impurities, and it requires time and fuel. In the event of incomplete combustion, it may even release soot and carbon monoxide to the environment.
- Another method to purify water is to add iodine. It is easy and effective in twenty minutes, and cheap, but this method is not suitable for long-term use. Even though iodine is able to kill the bacteria present in water, it might be toxic if too much iodine is added. Furthermore, it does not remove chemicals such as fertilisers and poisons. Pregnant women and children should avoid purification with iodine.
- The third method to purify water is to leave a clear water bottle in the sun for a day or two. Bacteria present in the water is destroyed via UV radiation and such method is cheap and convenient. However, this also depends on the weather conditions as the UV radiation from the Sun has to be strong enough for this method to work.
Question 2. Explain why desalination techniques, despite proven technological effectiveness, are not used more widely to produce potable drinking water.
Answer: Desalination techniques such as distillation and reverse osmosis are effective but very expensive and inconvenient due to high energy consumption and possible pollution to the environment. This is because large quantities of brine is released and has to be treated properly before being returned back to the ocean.
Question 3. Water quality in a chemical engineering building on campus was continuously monitored because testing indicated water from drinking fountains in the building had dissolved lead levels above those established by NEA.
- What is the likely major source of the lead in the drinking water?
- Do the research activities carried out in this chemistry building account for the elevated lead levels found in the drinking water? Explain.
Answer:
- The likely major source of the lead in the drinking water is from the corrosion of lead pipes in the building.
- Yes. Contaminated air, water, dust, food or consumer products due to exposure lead can all lead to an increase in lead levels found in the drinking water. It is also possible that the research activities involve lead as well.
Question 4. Some vitamins are water-soluble, whereas others are fat soluble. Would you expect either or both to be polar compounds. Explain.
Answer: No. Only vitamins that are water-soluble are polar compounds as it is able to form non-covalent bonds such as hydrogen bonding with water. Fat-soluble compounds are not soluble in water as it would form hydrophobic interactions with one another and would not form bonds with water molecules. Hence, it would repel from water as “like dissolves like”.
The Impact of Thalidomide On Society
Photo: Taken from http://www.wikiwand.com/de/Gr%C3%BCnenthal_(Unternehmen)
Origin of Thalidomide
Thalidomide was created by a family-owned pharmaceutical company, Chemie Grünenthal (Thalidomide Victims Association of Canada, 2017). Chemie Grünenthal was the first company to introduce Penicillin into the German market in the 1940s while in the 1960s, it introduced Thalidomide into the market.
Chemie Grünenthal first obtained a twenty-year patent for Thalidomide in 1954 and subsequently started clinical trials for the drug. Researchers at the company soon found out that the drug was rather effective in relieving morning sickness for pregnant mothers. Thalidomide was then marketed as Contergan® in the German market and was taken by many expectant mothers.
Beside West Germany where Thalidomide was first invented, Thalidomide was also distributed in the UK as Distaval, in Canada, and in the United States albeit without much success due to disapproval by the US Food and Drug Administration.
Impact of Thalidomide
Thalidomide was promoted as a ‘wonder drug’ to treat a range of conditions including headaches, insomnia and depression. It was popular because it was atoxic and so it was impossible to overdose on it (Thalidomide Society, N.A.). But long-term ingestion of the drug will result in irreversible peripheral neuritis, which is damage to the peripheral nerves, causing numbness, weakness and pain in the hands and feet (Mayo Clinic, 2017). The main impairments caused by thalidomide affect the limbs and are usually bilateral – either both arms or both legs or all four limbs (Thalidomide Society, N.A.).
Due to the absence of a census, there has not been an accurate number of fatalities resulted by Thalidomide. But according to the Thalidomide Victims Association of Canada, there were between ten and twenty thousand students born disabled as a result of the drug Thalidomide. The drug was responsible for deformities in children born by expectant mothers who consume it during their third trimester.
Impact of Thalidomide in Singapore
According to FFDN, the Thalidomide Association of Sweden , there is a personal anecdote of a lady born in the 1960s with disabilities in Singapore. She believed her defects at birth were a result of the consumption of Thalidomide by her mother during pregnancy. In her words, ‘there was an outbreak of infants born with deformed or missing limbs’. She received treatment as an infant which includes several operations and many hours of therapy to straighten her limbs. Growing up, she learnt how to walk with the help of callipers and walking frame.
Henceforth, against this personal narrative, there may perhaps be other children born with defects in the same period in Singapore which may have been left out of official records. Thus, Thalidomide did affect Singaporeans in the 1960s, although it is hard to estimate its impact.
Source: http://www.thalidomide.org/web/facts/
Chemistry Concept of Thalidomide
Researchers later realized that the problem lied in the fact that thalidomide was being provided as a mixture of two different isomeric forms.
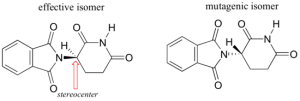
Diagram 1: Two isomers of Thalidomide
Both isomeric forms have the same molecular formula and the same atom-to-atom connectivity. Both isomers differ in the arrangement of the three-dimensional space about one tetrahedral, sp3-hybridized carbon. Hence, the two forms of thalidomide are known as stereoisomers. Tetrahedral carbons with four different substituent groups are called stereocenters as indicated in the diagram above
AE 6
Question 1. Mammoth Cave National Park in Kentucky is in close proximity to the coal-fired electric utility plants in the Ohio Valley. Noting this, this National Parks Conservation Association (NPCA) reported that this national park had the poorest visibility of any in the country.
- What is the connection between coal-fired plants and poor visibility?
- The NPCA reported ” the average rainfall in Mammoth Cave National Park is 10 times more acidic than natural.” From this information and that in your text, estimate the pH of rainfall in the park.
Answer:
- Coal-fired plants releases sulfur dioxide. This results in sulfate particles to be present in the atmospheric air near the plant, and thus contributing to the poor visibility at the park.
- Generally, rainfall is of pH range of 5-6. Therefore, given that the average rainfall in Mammoth Cave National Park is 10 times more acidic than natural, its rainfall should be in the pH range of 4-5.
Question 2. Here are examples of what an individual might do to reduce acid rain. For each, explain connection to producing acid rain.
- Hang your laundry to dry it.
- Walk, bike, or take public transportation to work.
- Avoid running dishwashers and washing machines with small loads.
- Add additional insulation on hot water heater and pipes.
- Buy locally grown produce and locally produced food.
Answer:
- Hanging your laundry to dry uses less energy as compared to using an electrical dryer to dry it.
- Walking, biking or taking public transport can reduce the amount of gasoline used.
- It uses less energy running dishwashers and washing machines only when they are fully loaded as opposed to running them in small loads.
- Adding additional insulation on hot water heaters and pipes can prevent heat loss and conserve energy. Therefore, electrical energy generated from the burning of coal in power plants can be conserved.
- Buying from locally grown produce and locally grown feed will greatly reduce the amount of gasoline used in transporting externally-grown produce. Therefore, less NO emission will be produced, contributing less to the acidity of the rain.
Question 3a. Give names and chemical formulas for five acids and five bases.
Answer:
Five acids:
- Nitric acid, HNO3
- Hydrochloric acid, HCl
- Sulfuric acid, H2SO4
- Phosphoric acid, H3PO4
- Carbonic acid, H2CO3
Five Bases:
- Sodium hydroxide, NaOH
- Potassium hydroxide, KOH
- Ammonium hydroxide, Nh4OH
- Magnesium hydroxide, Mg(OH)2
- Calcium hydroxide, Ca(OH)2
Question 3b. Name three observable properties generally associated with acids and bases.
Answer:
Properties of Acid:
- Taste sour
- Turn litmus paper red
- Release carbon dioxide from a carbonate.
Properties of Base:
- Taste bitter
- Turn litmus paper blue
- Slippery feel in water
Question 4. The concerns of acid rain vary across the globe. Many countries in North America and Europe have websites dealing with acid rain. Either search to locate one (”Canada, acid rain”) or use these links to websites in Canada, the UK, or Europe. What are the issues in Singapore? Does the acid deposition originate outside or inside the Singapore’s borders?
- http://www.ec,gc,ca/
- http://www.ukawmn.ucl.ac.uk/
- http://www.grida.no/
- http://www.nea.com.sg /
Answer: One issue that Singapore is facing is the impact of biomass burning on rainwater acidity and composition. Large scale forest fires in Indonesia, to clear land and make way for new harvest, are being set from August to October every year. This process releases large amount of gaseous and particulate pollutants into the atmosphere, and adversely affected the air quality in Southeast Asia region. A study done in 1997 revealed that major inorganic and organic ions (SO42-, NO3– and NH4+) were detected in the collected rain samples, and the acidity of this rain were between the pH values of 3.79 to 6.20. The decrease in pH of precipitation in response to the increased concentration of acids is marginal, due to the neutralisation of acidity by NH3 and CaCO3.
AE7
Question 1. When Styrofoam packing peanuts are immersed in acetone (the primary component in some nail-polish removers), they dissolve. If the acetone is allowed to evaporate, a solid remains. The solid still consists of Styrofoam, but now it is solid and much denser. Explain.
Hint: Remember that Styrofoam is made with foaming agents.
Answer: When acetone dissolves the polymer (polystyrene), gas trapped in the foaming agent is allowed to escape and the polymer collapses on itself. Given that density = mass/volume, when the gas is removed from the polymer, volume decreases and density increases. Hence, the polymer becomes more dense.
Question 2. Consider Spectra, Allied- Signal Corporation’s HDPE fiber, used as liners for surgical gloves. Although the Spectra liner has a very high resistance to being cut, the polymer allows a surgeon to maintain a delicate sense of touch. The interesting thing is that Spectra is linear HDPE, which is usually associated with being rigid and not very flexible.
- Suggest a reason why branched LDPE cannot be used in this application.
- Offer a molecular level reason for why linear HDPE is successful in this application.
Answer:
- Branched LDPE cannot be used in this application as it does not have required strength to act as liners of surgical gloves.
- Linear HDPE is successful in this application as a thin liner of it allows sufficient flexibility while providing the required strength for surgical gloves.
Question 3. When you try to stretch a piece of plastic bag, the length of the piece of plastic being pulled increases dramatically and the thickness decreases. Does the same thing happen when you pull on a piece of paper? Why or why not? Explain on a molecular level.
Answer: When a piece of plastic is stretched, the strip narrows and “necks down”. The molecules become aligned parallel to each other and in the direction of the pull. This alteration of the 3D structure is irreversible, and if the pulling continues, the plastic breaks. When the same pulling force is applied to a piece of paper, the paper tears instead of stretching. This is because paper is made up of cellulose molecules which form cross-linkers and are held more rigid in place. As such, the molecules are not able to be aligned parallel to each other to be stretched.
Question 4. A Teflon ear bone, a Fallopian tube, or heart valve? A Gore-Tex implant for the face or to repair a hernia? Some polymers are bio-compatible and now used to replace or repair body parts.
- List four properties that would be desirable for polymers used within the human body.
- Other polymers may be used outside your body, but in close contact with it. For example, no surgeon is needed for you to use your contact lenses- you insert, remove, clean and restore them yourself. From which polymers are contact lenses made? What properties are desirable in these materials? Either a call to an optometrist or a search on the Web may provide some answers.
- What is the difference in the material used in ”hard” and ”soft”contact lenses? How do the differences in properties affect the ease of wearing of contact lenses?
Answer:
- The benefits for polymers used within the body should far outweigh any risks. Two main properties of the polymers are being stable over time of the intended use and being non-toxic to the body. The polymers used in the body should also not be able to react with the bodily fluids or dissolve in them, and the ease of implantation should be considered.
- There are several different types of contact lenses on the market and each uses a different type of polymer. Polymathy methacrylate (PMMA), one of the earliest polymers used for rigid gas permeable lenses, is structurally similar to Lucite and plexiglas. Silicone-acrylate materials now are more commonly used under trade names such as Kolfocon. Newer rigid gas permeable (RGP) polymers contain fluorine: flour-silocone-acrylate polymers and flour-silicones.
- Hard contact lenses are typically made of PMMA, a rigid non-gas permeable plastic. The soft contact lenses that replaced them are made of silicone, which is flexible and allows oxygen to reach the eye. Due to such properties, the soft lenses tend to be more comfortable.
AE9
Question 1. In allergy sufferers, histamine causes runny noses, red eyes, and other symptoms. Here is its structural formula.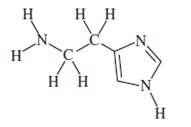
- Give the chemical formula for this compound.
- Circle the amine functional groups in histamine.
- Which part(s) of the molecule make the compound water soluble?
Answer:
- C5H9N3

- Amine functional group only.
Question 2. Antihistamines are widely used drugs for treating symptoms of allergies caused by reactions to histamine compounds. This class of drugs competes with histamine, occupying receptor sites on cells normally occupied by histamines. Here is the structure for a particular antihistamine.
- Give the chemical formula of this compound.
- What similarities do you see between this structure and that of histamine (shown in the previous question 1) that would allow the antihistamine to compete with histamine?
Answer:
- C16H21N3
- Pyridine
Question 3. Consider this statement. “Drugs can be broadly classed into two groups: those that produce a physiological response in the body and those that inhibit the growth of substances that cause infections.” Into with class does each of these drugs fall?
- aspirin
- morphine
- (Keflex) antibiotic
- estrogen
- amphetamine
- penicillin
Answer:
| Drugs that produce a physiological response in the body | Drugs that inhibit the growth of substances that cause infections |
| Aspirin, Estrogen, Morphine, Amphetamine | Penicillin, (Keflex) antibiotic |
Question 4. Herbal or alternative medicines are not regulated in the same way as prescription or OTC medicines. In particular, the issues of concern are identification and quantification of the active ingredient, quality control in manufacture, and side effects when the herbal remedy is used in conjunction with another alternative or prescription medicine.
- What do you think is the evidence from herbal supplement manufacturers that address these issues?
- Do you know anything about Singapore’s legislation on the topic?
Answer:
- Herbal supplement manufacturers may attain International Organisation for Standardization (ISO) Certification or the Certificate of Pharmaceutical Products (CPP) recommended by the World Health Organisation (WHO). Content of CPP should include the exporting (certifying) country, importing (requesting) country, recommended dosage and composition of products (active ingredients). Including the license holder details and date of issue of the product license can also address issues concerning the herbal medications.
- In Singapore, there is a Health Products Act in which manufacturers are required to provide information concerning its products and active ingredients. Manufacturers are also required to be licensed from Health Sciences Authority (HSA).







_logo.svg/440px-Gr%C3%BCnenthal_(Unternehmen)_logo.svg.png)


