- (a) C5N3H9
(b)
(c)
- (a) C16H21N3
(b) The similarities are the nitrogen at the end of the carbon chains, and the N-C-N sequence. The spatial arrangement of these two molecules are similar as well.
-
Physiological response in the body Inhibit the growth of substances that cause infection Morphin (Keflex) antibiotic Oestrogen Penicillin Amphetamine Aspirin
- (a) The manufacturers should be licensed, and they should display all important information of the product on its packaging. Consumers should be able to understand the ingredients and usage of the product.(b) The product label should be prominently displayed on the product during its sale. The use of leaflets and hang tags in appropriate formats will be allowed.The following information must be included as well:
-Name of the health supplement product
-Names and quantities of all the active ingredients
-Product indications
-Instructions on proper usage
-Pack size
-Batch number
-Expiry date
Application Exercise 7
1. Styrofoam contains pores which traps air within them. Once styrofoam is dissolved in acetone, the trapped air are released and therefore, large amount of styrofoam is able to dissolve in small amounts of acetone. However, the dissolved styrofoam remains in the acetone mixture. Hence, after the removal of acetone, the solid obtained is the solid itself and of higher density.
2a. The more branching it has, the higher cross-linking will lead to a stiffer material which will not be flexible. When the LDPE is stretched, the material narrows and necks down. The molecules align parallel to each other and in the direction of pull. The alteration is non-reversible and the material will break if the pulling continues. Hence, this is not very ideal for glove lining as LDPE does not have the desired strength.
2b. HDPE on the other hand is much harder and stronger than LDPE. Also, the weak extensive dispersion forces in HDPE allows the molecules to slip past one another and thus allow the material to stretch for a considerable length without breaking.
3. No, the same thing will not happen to paper. The weak but extensive dispersion forces in plastic also allow the molecules to slip pass one another allowing the plastic to stretch and neck. This alteration of the three-dimensional structure is not reversible, and if the pulling continues, the plastic breaks. Whereas for paper, it is made from cellulose. As cellulose is a rigid molecule, they are unable to slide pass each other and hence, the molecule are not free to become align with each other and thus, they are less flexible as compared to plastic.
4a. The four properties are: (1) non-toxic (2) non-immunogenicity (3) low-cost (4) degradability.
4b. For soft contact lenses, Silicon Hydrogel are used. For hard contacts lenses, polymethyl methacrylate (PMMA or Perspex/Plexiglas) are used. The desirable properties of contact lenses are durability, transparency and inert to chemicals on the eye surface. The lenses have to be non-toxic, comfortable and must be able to fit the shape of the eye.
4c. Hard contact lenses made of PPMA are usually rigid and non-permeable to gases whereas soft contact lenses are hydrophilic and has high oxygen permeability and flexible, making it more comfortable.
Application Exercise 6
1a. Coal-fired plants release SOx which reacts with water to form sulfuric acid. These many molecules of sulfuric acid forms tiny droplets of aerosols that do not absorb sunlight but instead reflect the sunlight, reducing visibility.
1b. pH of natural rainwater is approximately 5.3. Hence, the pH of the average rainfall in Mammoth Cave National Park should be approximately 4.3.
2. NOx and SOx are main contributors of acid rain.
2a. By hanging your laundry outside to dry, this reduces the electricity consumption as the electronic dryer is not used. Hence, less energy is needed to be generated from power plants, resulting in burning of less fossil fuels such as coal. Less SOx and NOx are produced, reducing acid rain formation.
2b. Less usage of automobiles will reduce consumption of fossil fuels hence reduce emissions of NOx from automobile vehicles.
2c. Same amount of energy is required to run full and small loads. Running dishwaters and washing machines with small loads will not optimise the energy usage. Reduce frequency of running dishwaters and washing machines will reduce the energy needed to be generated from power plants, hence less SOx and NOx will be produces.
2d. Adding additional insulation will reduce the heat loss of hot water to surroundings. Hence, less energy will be needed to maintain the hot temperature of water heaters and pipes, decreasing production of NOx and SOx from power plants.
2e. Consuming local grown goods will reduce the need for transportation of imported goods across further distances. Thus, less emissions (NOx) as there is less usage of transportation vehicles.
3a. Acids:
- Hydrochloric acid, HCl.
- Sulfuric acid, H2SO4.
- Ethanoic acid, CH3COOH.
- Nitric acid, HNO3.
- Phosphoric acid, H3PO4.
Bases:
- Sodium hydroxide, NaOH.
- Potassium hydroxide, KOH.
- Barium hydroxide, Ba(OH)2.
- Lithium hydroxide, LiOH.
- Calcium hydroxide, Ca(OH)2.
3b. Acids: Sour taste, turns blue litmus paper red, corrosive
Bases: Slippery feel, bitter, turns red litmus paper blue.
4. Acid rain can be caused by a few factors:
- Pollutants such as SOx and NOx are produced from places such as power stations and oil refineries.
- Harmful emissions such as NOx from automobiles.
- Open burning of waste materials
The pollutants mentioned above dissolve in rainbow to produce acid rain.
Apart from the factors mentioned above, slash and burn from neighbouring countries such as Indonesia may contribute to haze and acid rain in Singapore. This is because pollutants such as NOx and SOx which build up in Indonesia are transported to Singapore under favourable wind conditions.
Application Exercise 5
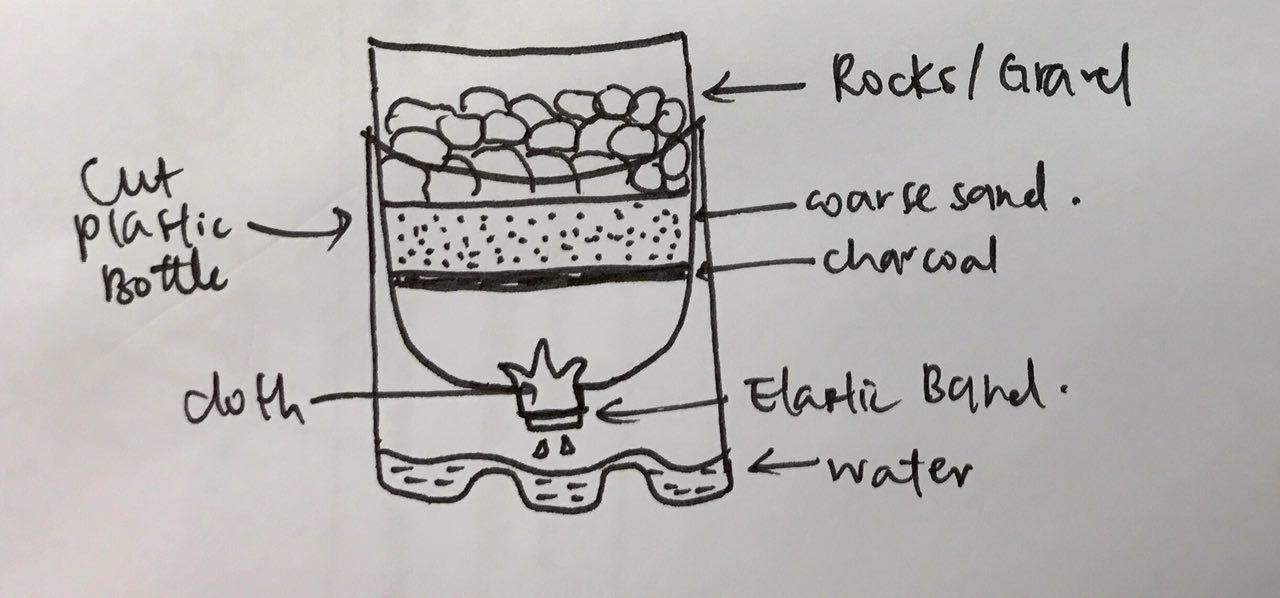
- Method 1: Filtration using available materials around us (e.g gravel/rocks, coarse sand, charcoal, cloth and a plastic water bottle). set up the filtration as shown in the diagram below.
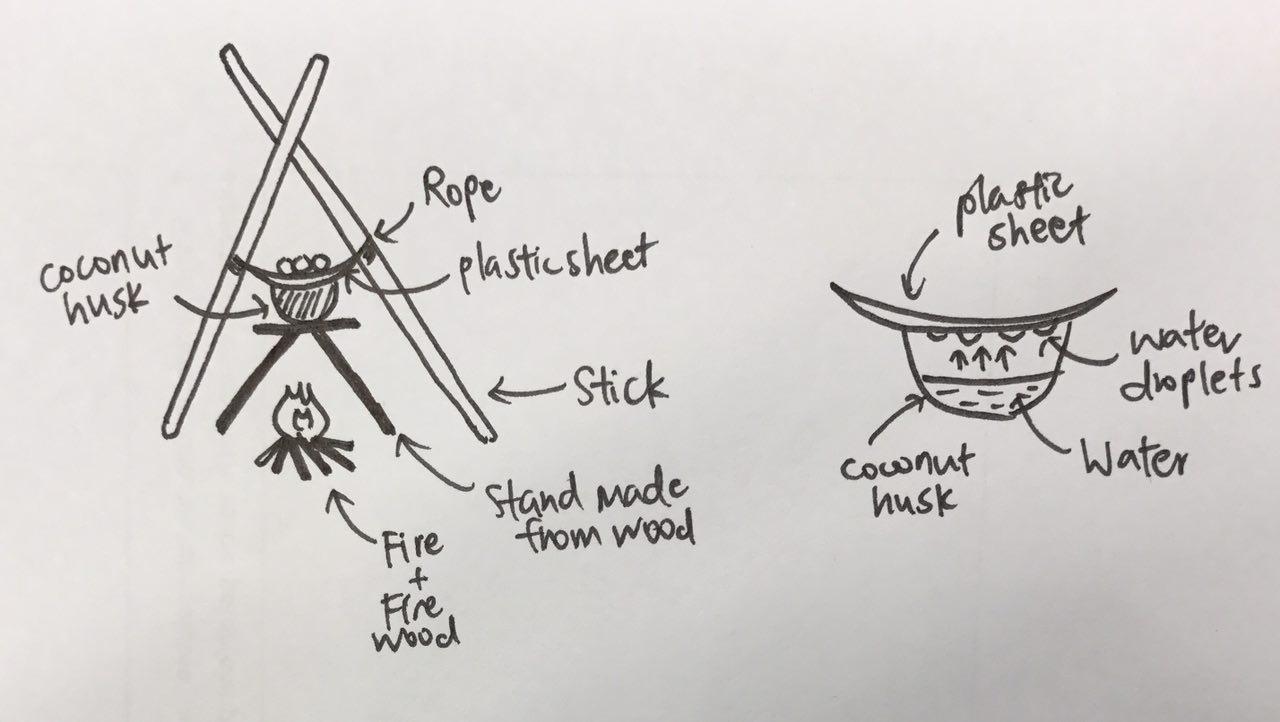
Method 2: Distillation. Set up distillation method as shown in the diagram below. Boil the water over a fire and collect the water droplets which condense on the plastic sheet. The water obtained is pure water.
Filtration is more cost and energy efficient whereas distillation is more energy consuming and non-economical. However, distillation produces water of higher purity.
Filtration is similar to the Microfiltration process in the purification process in NEWater. Distillation is similar to the desalination of sea water to obtain pure water.
2. Despite proven technological effectiveness, desalination techniques such as reverse osmosis and distillation are considered to be expensive and energy consuming, hence it is only practiced in richer countries.
3a. The likely major source of the lead in the drinking water maybe due to the corrosion of lead pipe, or solder from the drinking fountains and water tanks.
3b. Probably not, because all of the research activities conducted in the building usually have strict practice and proper waste disposal cans for the disposal of heavy metals, it is very unlikely that the research activities conducted contributes to the elevated lead levels in drinking water. However, human error may occur sometimes.
4. According to the “like dissolves like” assumption, we expect that the water-soluble vitamins are polar as they are soluble in polar solvents like water. On the other hand, the fat-soluble vitamins are non-polar as they are soluble in non-polar molecules like fats(hydrocarbon chains of fats are non-polar).
Application Exercise 4
- Greenhouse gases trap the Earth’s infrared radiation. So, the atmosphere keeps the Earth warm.
- Global warming is a long term change. There may be insignificant and inconsistent increases in temperature change in a specific year. However, the increases in temperature change over a period of time may then be more significant to determine that global warming has really occured. So, the statement is not completely accurate because “this winter” only refers to a specific year.
- There is water vapour in the air. So, the radar using water molecule rotation will be affected by noise from the water vapour, especially on rainy days. The absorption of microwave radiation by water in the atmosphere interferes with the detection of intended objects.
Also, microwave radiation of a wavelength triggers the rotation of water molecules. This heats up the air around the machine, leading to diseases caused in the operators of the radar. - Air quality will affect us in the short run. Air pollution caused by vehicle emissions (NO2, CO, etc) can be hazardous to our health (cause respiratory problems). Global warming will affect us in the long run. It results in an increase in temperature on Earth that leads to rises in sea levels. This will take a long time for it to have a significant impact. So, even though there is no significant impact on us now, we may experience the accumulated effects in the future.
Application Exercise 3
1a. Exothermic. As the burning of briquette produces heat energy.
1b. Endothermic. Heat energy is required to break the water molecules in order for it to enter the gaseous state and evaporate from our skin.
1c. Exothermic. Heat energy is required to break the lattice energy of the ice in order for the ice to melt.
2. The bond strengths of the reactants has to be relatively weaker as to have a lower activation energy for the reaction. In contrast, the bond strengths of the products need to be relatively stronger in order for more heat to be released in the process so as to create an exothermic reaction that would make for a good explosion.
3. Heat is the energy that flows from a hotter to colder object whereas temperature determines the direction of the heat flow.
For example, imagine putting some ice into a cup of hot tea. The ice would melt almost instantly due to the heat from the hot tea. But the temperature of the hot tea would drop, showing the direction of the heat flow from the tea to the ice cubes.
4a. It has a knocking characteristic of 98% isooctane and 2% n-heptane. As a higher grade premium gasoline, it has higher octane rating than other blends sold at other gasoline stations and hence is more resistant to knocking.
4b. The octane rating does not produce any information regarding the presence of any oxygenates and such details should be available on other parts such as labels around the pump.