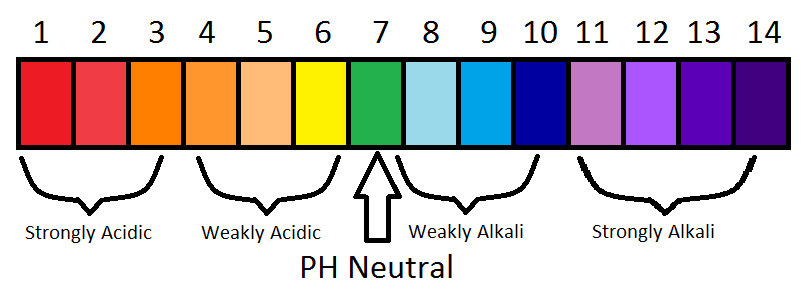
pH is the measurement of the concentration of hydrogen ions/[H+] present in a solution. It is measured using a pH meter or universal indicator and the colours varies with pH. It has a value of 0 – 14 (0 being the most acidic, 7 being neutral and 14 being the most basic).
A neutral solution contains equal concentration of hydrogen ions and hydroxide ions. The pH of the solution is 7 and gives a green solution with a pH indicator.
[H+] = [OH–] (pH = 7)
An acidic solution contains a higher concentration of hydrogen ions than hydroxide ions. The pH of the solution is less than 7 and the colour ranges from red to yellow depending on the acidity of the solution. It also turns blue litmus paper red.
[H+] > [OH–] (pH < 7)
A basic solution contains a higher concentration of hydroxide ions than hydrogen ions. The pH of the solution is more than 7 and the colour ranges from blue to violet depending on the basicity of the solution. It also turns red litmus paper blue.
[H+] < [OH–] (pH > 7)
Therefore, by adding an acid or a base to a solution, the pH of the solution can be adjusted.