1a) A charcoal briquette burning is an exothermal process. This is because heat is released into the surroundings during the process, which is evident from how we feel warmth coming from it.
 1b) Water evaporating from our skin is an endothermic as heat is absorbed by water, breaking its intermolecular bonds to form water vapour. When the water evaporates, it removes heat from our skin. This is why our hand feels cool and this is also how sweat cools us down.
1b) Water evaporating from our skin is an endothermic as heat is absorbed by water, breaking its intermolecular bonds to form water vapour. When the water evaporates, it removes heat from our skin. This is why our hand feels cool and this is also how sweat cools us down. 1c) Ice melting is similar to water evaporating. Hence it is endothermic as heat is absorbed by the ice to weaken the intermolecular bonds in ice to form liquid water.
1c) Ice melting is similar to water evaporating. Hence it is endothermic as heat is absorbed by the ice to weaken the intermolecular bonds in ice to form liquid water. 
2) A good explosion requires a rapid release of a large amount of energy, with the generation of high temperatures. Hence it is logical that the reaction is highly exothermic. For an exothermic reaction, the relative bond strength of products needs to be higher compared to the reactants, so that the difference in potential energy can be released as energy. This is illustrated in the figure below.
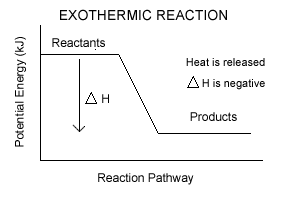
3) Heat is the amount of energy flowing from one body to another spontaneously due to the temperature difference. Heat is the reason why an ice-cream melts under the sun.
 Temperature is a measure of the warmth or coldness of an object or substance. Thermometers are a tool to measure temperature.
Temperature is a measure of the warmth or coldness of an object or substance. Thermometers are a tool to measure temperature.
4a) An octane rating of 98 indicates that the premium gasoline has a knocking characteristic of 98% of isooctane and 2% of heptane. The higher the octane rating, the greater the gasoline’s ability to prevent knocking.
4b) The octane rating does not provide information on whether the fuel contains oxygenates. However, oxygenates in fuel are known to increase octane number and therefore reduce knocking. 
