Q1) In allergy sufferers, histamine causes runny noses, red eyes, and other symptoms. Here is its structural formula.
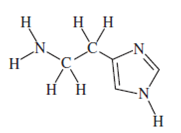
a. Give the chemical formula for this compound.
Answer: C5H9N3
b.Circle the amine functional groups in histamine.
Answer: 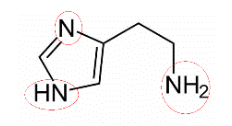
c. Which part (or parts) of the molecule make the compound water-soluble?
Answer: The amine parts, as they form hydrogen bonds with water.
Q2) Antihistamines are widely used for drugs for treating symptoms of allergies caused by reactions to histamine compounds. This class of drug competes with histamine, occupying receptor sites on cells normally occupied by histamine. Here is the structure for a particular antihistamine.
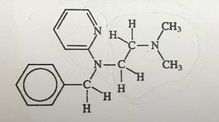
a. Give the chemical formula for this compound.
Answer: C16H21N3
b. What similarities do you see between this structure and that of histamine (shown in the previous question 1) that would allow the antihistamine to compete with histamine?
Answer: 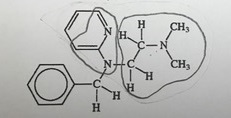
When histamine and antihistamine are both present at the same time, antihistamine cancels the effects of histamine. This is because once antihistamine binds to the receptor site, it blocks the effects of histamine as histamine is unable to bind to the receptor site. No chemical messengers would be produced by histamine.
Q3) Consider this statement. “Drugs can be broadly classed into two groups: those that produce a physiological response in the body and those that inhibit the growth of substances that cause infections.” Into which class does each of these drugs fall into?
A) aspirin B) morphine C)(Keflex) antibiotic D) estrogen E) amphetamine F) penicillin
Answer:
Produce a physiological response in the body: A, B, D, E
Inhibit the growth of substances that cause infections: C, F
Q4) Herbal or alternative medicines are not regulated in the same way as prescription or OTC medicines. In particular, the issues of concern are identification and quantification of the active ingredient, quality control in manufacture, and side effects when the herbal remedy is used in conjunction with another alternative or prescription medicine.
A) What do you think is the evidence from herbal supplement manufacturers that address these issues?
A) Do you know anything about Singapore’s legislation on the topic?
Answer: In Singapore, health supplements can be imported and sold without a license from Health Science Authority (HSA). The HSA provides guidelines, which should be followed.
According to HSA, there are several acts in Singapore’s legislation that cover health supplements:
A. Medicines Act (Chapter 176) & its Subsidiary Legislation especially:
i. Medicines (Prohibition of Sale & Supply) Order;
ii. Medicines (Traditional Medicines, Homoeopathic Medicines and Other
Substances) (Exemption) Order;
iii. Medicines (Non-Medicinal Products)(Consolidation) Order;
iv. Medicines (Labelling) Regulations;
v. Medicines (Medical Advertisements) Regulations;
vi. Medicines (Licensing, Standard Provisions & Fees) Regulations
B. Medicines (Advertisement & Sale) Act (Chapter 177)
C. Sale of Drugs Act (Chapter 282) & its Regulations especially:
i. Sale of Drugs (Prohibited Substances) Regulations;
ii. Sale of Drugs (Prohibited Drugs) (Consolidation) Regulations;
iii. Sale of Drugs (Rhodamine B) Regulations 1993
D. The Poisons Act (Chapter 234) & The Poisons Rules
References
- Health Supplements Guidelines [PDF]. (2015, February). http://www.hsa.gov.sg/content/dam/hsa/hprg/complementary_health_products/overview_framework_policies/health_supplements/hsguidelines.pdf