Question 1
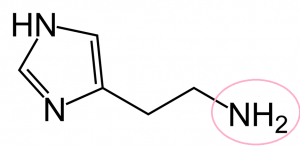
a. C5H9N3
b.
c. Amine
Question 2
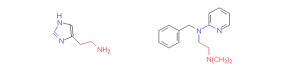
a. C17H21NO
The similarities are as below:
(a) a two-carbon chain with a nitrogen at the end (marked in red)
(b) a three-atom sequence of N-C-N (marked in blue).
(c) The flat 5-ring of histamine seems to be similar to the flat 6-ring of the antihistamine.
Due to the similarities in structure, the antihistamine will compete with histamine to bind to the histamine receptor sites on cells.
Question 3
| Drugs that produce physiological response in the body | Drugs that inhibit the growth of infectious substances |
| Aspirin | (Keflex) Antibiotic |
| Morphine | Penicillin |
| Estrogen | |
| Amphetamine |
Question 4
a.
The herbal supplement manufacturers are permitted to say, for example, that a particular herbal supplement addresses a nutrient deficiency, supports health, or is linked to a particular body function (like immunity or heart health). Such a claim must be followed by the words, “This statement has not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.” Besides, the herbal supplement manufacturers must follow certain good manufacturing practices to ensure the identity, purity, strength, and composition of their products. If a product is found to be unsafe or otherwise unfit for human consumption, the authorities may take enforcement action to remove the product from the marketplace or work with the manufacturer to voluntarily recall the product.
Reference: https://ods.od.nih.gov/HealthInformation/DS_WhatYouNeedToKnow.aspx
Products like herbs are sometimes tainted with germs, pesticides, or toxic heavy metals. Other supplements do not contain what’s listed on the label. Still, others contain more or less than the amount of the herb listed on the label. And many have ingredients that aren’t listed on the label at all.
This problem extends beyond the supplement makers and sellers. Some herbal suppliers (those who grow, harvest, or sell the crops) may mix or even substitute their crops with less expensive or more readily available plants. There’s also the problem of accidental contamination when one plant grows in with others, as well as cases of mistaken identity (when one plant looks like another). Given the global market, all of these problems can make it harder for a company to be sure that what they thought they were buying to make supplements is actually the herb they wanted.
b. There are limits for the safety and quality levels for heavy metals and microbial in the supplement.
“The product label should be prominently and conspicuously displayed on the product at the point of sale. Where the size, shape or nature of the final product or package does not permit the full listing of labelling information, the use of inserts, leaflets, hang tags, in appropriate format, will be allowed. However, the name of the product, the recommended dosage, the batch reference and relevant precautionary statements should be displayed on the final product or package.”
Reference: http://www.hsa.gov.sg/content/dam/HSA/HPRG/Complementary_Health_Products/Overview_Framework_Policies/Health_Supplements/HSGuidelines.pdf