Chemical Concepts of Acid Rain
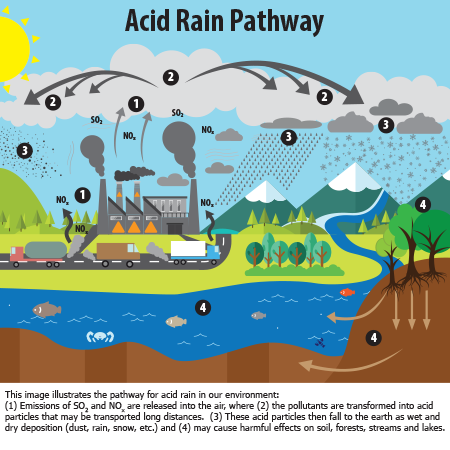
Figure 1: What is acid rain? ( United States Environmental Protection Agency, n.d.)
Acid rain is acidic precipitation (usually as rain) that has increased levels of hydrogen ions. It can be caused by the emission of sulphur dioxide, nitrogen oxide and other pollutants which react with water molecules in the atmosphere to become acidic. When it is very acidic, it can cause harmful ecological effects such as in rivers and lakes. This can make the water more acidic, affecting the aquatic life and the environment as a whole.
To understand acid rain, we need to first know what an acid is, how does the neutralisation process work, what forms acid rain, where do the reactants come from, and finally, how does acid rain form. In this post, we shall address these topics.
Content
1. Acid and Base
2. Introducing pH
3. Neutralisation
4. Formation of Acid rain
5. Sulfur Dioxide
6. Nitrogen Oxides
7. The Nitrogen Cycle
8. References
1. Acid and Base
Acids are generally sour, corrosive and turn blue litmus paper red [1]. More formally, they are defined as a species that dissociate to produce H+ ions in solution. For example, acid HA dissociates in water according to the equation:
HA (aq) → H+ (aq) + A– (aq)
When H+ ions are present in water, they readily react with water molecules to form the hydronium ion, H3O+. As such, a more appropriate equation for HA dissociation in water would be:
HA (aq) + H2O (l) → H3O+ (aq) + A– (aq)
How do we know if an acid is strong? The strength of an acid is judged based on the extent of dissociation in water. A strong acid where completely dissociates H+ in water, while weak acids partially dissociate H+ in water.
Bases on the other hand, have a bitter taste, is soapy, and turns red litmus paper blue. They are defined as a species that dissociate to produce OH– ions in solution. For example, base BOH dissociates in water as below:
BOH (aq) → OH– (aq) + B+ (aq)
Ammonia, NH3 is one of the exceptions to this definition. It is a base even though it does not have OH– to dissociate. The dissociation of ammonia in water is shown below:
NH3 (aq) + H2O (l) → NH4OH (aq)
NH4OH (aq) → NH4+ (aq) + OH– (aq)
Similar to acids, strength of bases are also defined by the extent of dissociation in water, with the terms strong and weak bases used to describe high and low extents of dissociation respectively.
In summary,
| Acid | Bases | |
| Definition | dissociates H+ in water | dissociates OH– in water |
| Physical Properties | Sour
Corrosive Turns blue litmus paper red |
Bitter
Soapy Turns red litmus paper blue |
| pH | <7 | >7 |
2. Introducing pH
So, what does it mean when we say that acids have a pH of less than 7, and bases have a pH of more than 7? Let us introduce the pH scale.
pH stands for the power of hydrogen, and it measures the acidity of a solution by using the concentration of H+ ions [H+]. Note that the square brackets means that we are referring to the concentration of the given substance inside the square brackets. A less commonly used measure for basicity is pOH, power of hydroxide, similar to that of pH.
In general,
In a neutral solution pH=7, [H+] = [OH–].
In an acidic solution pH<7, [H+] > [OH–]
In a basic solution pH>7, [H+] < [OH–]
Why is this so?
As defined above, acids dissociate to produce a certain concentration of H+ ions. Likewise for bases, they produce a certain concentration of OH– ions. The value of pH and pOH can simply be calculated as:
pH = -log10[H+]
pOH = -log10[OH–]
Since the pH and pOH scales are calculated to log base 10, it would mean that every 1 pH/pOH value change would represent a power of 10 change in [H+]/[OH–].
Also, an important relationship to note is the ion-product constant of water, Kw, defined as
Kw = [H+][OH–] = 1 x 10^-14
Therefore, pH + pOH = 14
This relationship allows us to calculate both pH and pOH given either [H+] or [OH–].
When the concentration of H+ is more than 1 x 10^-7, pH will be less than 7, which is the case for acids. For example if concentration of H+ is 1* 10^-6, pH= 6. This also means that when the concentration of OH– is more than 1 x 10^-7, pH will be more than 7. A pH of 7 shows that the concentrations of H+ and OH– are equal, hence the solution is neutral.
3. Neutralisation
Since we have introduced acids and bases, we shall talk about a common process between the both of them. What happens if we mix an acid with a base?
When an acid is added to a base, neutralisation occurs. Neutralisation reactions are processes where acids react with bases to produce an ionic salt and H2O, in the general molecular equation:
HA (aq) + BOH (aq) → AB (aq or s) + H2O (l)
The products are no longer acidic or alkaline, and are neutral.
How is this relevant to acid rain? It is important to note that acid raid is harmful because of it’s acidity. Hence through neutralisation, we can remove the acidity of the rain.
4. Formation of Acid Rain
Acid rain is caused by a chemical reaction between sulfur dioxide and nitrogen oxides in the air [2]. These substances mix and react with water, oxygen, and other chemicals to form acidic pollutants, known as acid rain.
Sulfur dioxide and nitrogen dioxide are acidic oxides and react with water to form acids [3].
Sulfur dioxide reacts with water to form sulfurous acid.
SO2(g) + H2O(l) -> H2SO3(aq)
Substances in the upper atmosphere then catalyse the reaction between sulfurous acid and oxygen to form sulfuric acid.
2H2SO3(aq) + O2(g) -> 2H2SO4(aq)
Similarly, nitrogen dioxide reacts with water to form a mixture of nitric acid and nitrous acid.
2NO2(g) + H2O(l) -> HNO3(aq) + HNO2(aq)
Substances in the atmosphere then catalyse the reaction between nitrous acid and oxygen causing the formation of more nitric acid.
2HNO2(aq) + O2(g) -> 2HNO3(aq)
In summary, both sulfuric acid and nitric acid are soluble in water and are the major acids present in acid rain. Where does sulfur dioxide and nitrogen oxide come from? We shall discuss this in the following parts.
5. Sulfur Dioxide
Sulfur dioxide is a colorless gas with brings along an irritating, choking odor.
Coal-fired power plants are the largest human-caused source of sulfur dioxide. Although natural processes like forest fires, biological decomposition and volcano eruptions also produce sulfur dioxide, emissions produced by human activity far surpasses that of nature. [3]
Sulfur dioxide is a key contributor to acid rain as explained through its chemical reactions below.
Coal naturally contains sulfur, and when coal is burned, the sulfur released combines with oxygen during combustion to form sulfur oxides:
S + O2 → SO2 (sulfur dioxide gas – poisonous)
Once in the air, the sulfur dioxide gas reacts with the oxygen molecules in the atmosphere to form sulfur trioxide; said process is aided by the dust and ultraviolet light:
2SO2 + O2 → 2SO3 (sulfur trioxide)
The sulfur dioxide and sulfur trioxide then reacts with water / water vapour in the atmosphere to form:
SO2 + H2O → H2SO3 (sulfurous acid – mist)
SO3 + H2O → H2SO4 (sulfuric acid – mist)
The rain drops that come in contact with these acidic mists will increase their acidity by a factor of up to 1000. This leads to the formation of acid rain. [5]
6. Nitrogen Oxides
Nitrogen oxides (NOx) are produced from reaction among nitrogen and oxygen during combustion of fuels, especially at high temperatures. Oxygen and nitrogen generally do not react at ambient temperatures, but at high temperatures, they undergo an endothermic reaction producing oxides of nitrogen. NOx gases are also naturally produced by lightning. [5] The highest NOx emissions are generally found in states with large urban areas, high population density and heavy automobile traffic.
Chemical reaction of nitrogen and oxygen during combustion:
N2 + O2 → 2NO (at high temperatures)
Once formed, NO is highly reactive and reacts with hydroxyl radical, oxygen and volatile organic compounds (VOC) to form NO2:
VOC + OH (radical) → A + O2 → A’ + NO → A’’ + NO2
A, A’ and A’’ are reactive intermediate species present in trace amounts. The production of acid rain requires a trace amounts of VOC in the atmosphere.
| Fuel | Emission of NOx 1) (10-3 kgNOx / kgfuel) |
|---|---|
| Oil | 3.0 |
| Kerosene | 3.0 |
| Coal | 4.5 |
| Propane | 2.3 |
| Gasoline | 27 3) |
| Hydrogen | 0 2) |
| Natural Gas | 1.0 |
| Butane | 2.3 |
| Wood, Birch 20% moisture content | 0.7 |
As seen from the table above, out of the various kinds of fuels used, gasoline produces the most concentrated amount of nitrogen oxides (significantly more, more than five times of the next highest emitter – coal). [6] Hence, the production of nitrogen oxides can be directly attributed to the combustion of gasoline.
NO2 gas in the atmosphere reacts with the hydroxyl radical to form nitric acid:
NO2 + .OH → HNO3 (nitric acid)
Overall:
4NO2(g) + 2H2O(l) + O2(g) → 4HNO (nitric acid)
7. Nitrogen Cycle
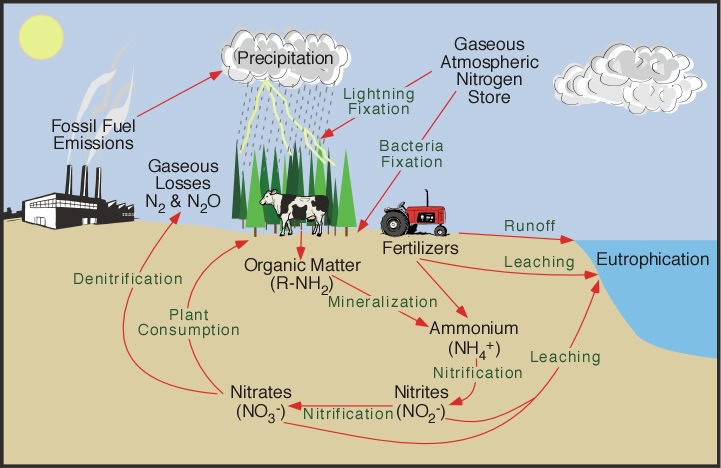
Figure 2: Nitrogen Cycle (Pidwirny, M.,2006.)
Another source of nitrogen dioxide comes from the nitrogen cycle.
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into various chemical forms as it circulates the atmosphere and terrestrial and marine ecosystems [7]. It consists of four processes, namely fixation, ammonification, nitrification and denitrification.
Process 1: Nitrogen Fixation
Nitrogen fixation is the process wherein N2 is converted to ammonium, or NH4+ by nitrogen-fixing organisms such as bacteria.This is done through a metabolic process, which is similar to breathing.
Other than nitrogen-fixing bacteria, some natural phenomena such as lightning, forest fires, and hot lava flows can cause the fixation of smaller, but significant, amounts of nitrogen. This is because these processes have high energy and can break the triple bonds of N2 molecules, hence leaving behind N atoms that can undergo transformation.
Human activities such as burning fossil fuels, using synthetic nitrogen fertilizers, and cultivating legumes also lead to nitrogen fixation.
Process 2: Nitrogen Ammonification
Organic N → NH4+
Decomposition is the process whereby nitrogen which has been incorporated into organic matter is converted back into inorganic nitrogen. This process is also called nitrogen mineralisation. Decomposers such as bacteria and fungi consume the organic matter of dead organisms and nitrogen within them is converted to ammonium. In the form of ammonim, the nitrogen can be used by plants or converted further into nitrate (NO3–) through the process called nitrification.
Process 3: Nitrification
NH4+ → NO3–
Nitrification is the process whereby ammonium is converted to nitrate (NO3–). The bacteria that carry out this reaction gain energy from it. Nitrification occurs only in oxygen-rich environments like circulating or flowing waters and the surface layers of soils and sediments.
Ammonium ions (NH4+) are positively charged and therefore are absorbed to negatively charged clay particles and soil organic matter. Hence, ammonium nitrogen cannot be removed from the soil via rainfall. However, the negatively charged nitrate ion is not held by soil particles and so can be washed out of the soil, leading to decreased soil fertility and nitrate enrichment of downstream surface and groundwater.
Process 4: Denitrification
NO3– → N2+ N2O
Denitrification is an anaerobic process that is carried out by denitrifying bacteria. It is the only nitrogen transformation process that removes nitrogen from ecosystems thus it balances the amount of nitrogen fixed by the nitrogen fixers described above.
Conversion of nitrate to dinitrogen takes place in the following sequence:
NO3– → NO2– → NO → N2O → N2.
Nitric oxide (NO) contributes to smog, and nitrous oxide (N2O) is a greenhouse gas, hence both gases are bad for the environment.
Once converted to dinitrogen, nitrogen is unlikely to be reconverted to a biologically available form because it is a gas and is rapidly lost to the atmosphere.
8.References
- United States Environmental Protection Agency. (n.d.). Retrieved 15 March 2017, from https://www.epa.gov/acidrain/what-acid-rain
- Acids, bases, and pH. (n.d.). Retrieved March 16, 2017, from https://www.khanacademy.org/science/chemistry/acids-and-bases-topic#acids-and-bases
- What causes Acid rain? (n.d.). Retrieved March 17, 2017, from https://www3.epa.gov/acidrain/education/site_students/whatcauses.html
- Formation of Acid rain. (2011, October 8). Retrieved March 17, 2017, from http://nsb.wikidot.com/c-9-3-2-10
- Sulfur dioxide and coal. (n.d.). Retrieved February 22, 2017, from http://www.sourcewatch.org/index.php/Sulfur_dioxide_and_coal
- Pidwirny, M. (2006). “The Nitrogen Cycle”. Fundamentals of Physical Geography, 2nd Edition. Retrieved 15 March 2017, from http://www.physicalgeography.net/fundamentals/9s.html
- Sulfur Dioxide, Sulfur Trioxide and Acid Rain: Pollution from Burning Coal. (2014, January 20). Retrieved February 22, 2017, from https://chemicalstatistician.wordpress.com/2013/04/07/sulfur-dioxide-sulfur-trioxide-and-acid-rain-pollution-from-burning-coal/
- NOx. (2017, February 14). Retrieved February 22, 2017, from https://en.wikipedia.org/wiki/NOx
- The Nitrogen Cycle: of Microbes and Men. (n.d.). Retrieved March 15, 2017, from http://www.visionlearning.com/en/library/Earth-Science/6/The-Nitrogen-Cycle/98