Implications of Acid Rain
As previously discussed in our previous post, acid rain is formed from nitrogen oxides and sulfur dioxide. Why is it important for us to know about acid rain? In this post, we are going to discuss the impact of acid rain on our environment, and hopefully highlight the significance of this topic.
In general, acid rain is harmful to our environment because it makes our water bodies inhabitable for aquatic life, damages forests, corrodes buildings, and worsen the air around us, thus affecting our health.
Content
- Damaging Lakes and Streams
- Damaging Forests
- Effects on Materials
- Haze and its effects on human health
1. Damaging Lakes and Streams
Acid rain damages lakes and streams because it makes water acidic. As acidic water flows through the soil into lakes and streams, it absorbs aluminium and carries it into the water bodies. The aluminium makes lakes and streams toxic, thus harming aquatic species [1]. In an interconnected ecosystem, when aquatic species are affected, organisms above and below the food chain will eventually be affected too. This includes us, the humans.
To highlight the significance of the damage to aquatic life, we shall take a look at how this increase in acidity affects trouts.

Figure 1: (Ophardt, E . Charles., 2003)
Let’s look at baby trouts. Looking at the top right picture and the bottom right picture from Figure 1, we observe that a pH of 5.0 and below would cause baby trouts to grow abnormally. Baby trout hatch in this condition will hardly survive in the environment, thus the population of trouts will decrease over time. Consequently, the disappearance of fish as predators and preys can lead to abnormal growth of some species such as bloodworm or water beetles which will resulted in an imbalance in the ecosystem.
In the long run, acidifying lakes and streams will make the water inhabitable and endanger the aquatic species. Due to the interconnections of the food chain, other species will also be affected due to a drastic change in number of predators and preys. How is this relevant to us? Eventually, humans will also be affected due to a decrease in supply of seafood, and the contamination of drinkable water. Thus, acid rain will eventually threaten our survival.
2. Damaging Forests

Figure 2: (Earthwatch, n.d.)
Acid rain damages forests. This is because acid rain removes essential nutrients and releases aluminium into the soil, thus trees cannot absorb water [3]. Furthermore, acid rain weakens the trees. This is because acid rain wears away the protective layers of the tree. The loss of the coating damages the leaves and creates brown spots [4]. When leaves have brown spots, they cannot photosynthesize and as a result, trees cannot receive food. Trees and plants also become more vulnerable to harsh weather, diseases and the attacks by insects.
There are many harmful consequences when trees are damaged. Firstly, species which live on trees will lose their habitats. As with the case of aquatic life in damaged lakes and streams, in the long run, the entire food chain will be affected. Since trees are the main source of oxygen, decreasing population of trees also mean that our oxygen supply is decreasing. In addition, trees absorb the greenhouse gas carbon dioxide. When the number of trees decline, more carbon dioxide will remain in the environment, thus global warming will be more severe. Overall, damaged lakes, streams and forests will reduce our biodiversity.
3. Effects on Materials
Not all acidic deposition is wet. Sometimes dust particles can become acidic as well, and this is called dry deposition.
Acid rain and dry acidic particles (like dust) can contain nitric acid and sulphuric acid. Man-made materials gradually degrade when exposed to unpolluted rain. Through long term exposure, acid rain can corrode infrastructure such as stone, metal and paint. When they come into contact with statues, buildings and other manmade structure, the acidic particles can accelerate the degradation process through damaging their surfaces.
This can lead to damaged materials that need to be repaired, replacement of damaged materials and loss of carved detail on buildings, statues, monuments and tombstone (marble, stone, metal, etc).
The repairing of acid rain corrosions and damages to buildings and monuments can even cost billions of dollar. However, for ancient monuments and buildings like the StoneHenge, its aesthetic and historical value may be altered over years of weathering and its original state can never be replaced.
Example 1: On Buildings
Acid rain contains sulphur dioxide. Both wet or dry forms of sulphur dioxide significantly increases the rate of corrosion on limestone, sandstone, and marble [5].
Acid Rain
SO2 + H2O –> H2SO3
SO3 + H2O –> H2SO4
Sulphuric acid can further react with the limestone in a neutralization reaction. Calcium sulphate/limestone (soluble is water) dissolves in water and the structure is corroded.
Limestone: CaCO3 + H2SO4 –> CaSO4 + H2CO3
H2CO3 –> CO2 gas + H2O
Example 2: Sculptures
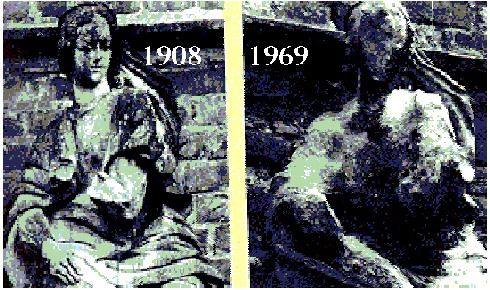
Figure 3: (teamsavetheworld, n.d.)
Many sculptures has been brought indoors to prevent the harmful corrosion from acid rain and preserving them.
It is similar to adding vinegar (acetic acid) to eggshell (calcium carbonate), which produces immediate bubbling (carbon dioxide) and eggshell is converted to carbonic acid. Over longer time, the eggshell (calcium carbonate) will be dissolved.
4. Haze and it’s Effects on Human Health
Figure 4: (SBCC Baby and Child Clinic, n.d.)
One of the more relevant harmful causes of acid deposition is from haze. Biomass burning accounts of an important source of airborne particulate matter (air pollution) in Asia, and this raises the potential for air pollution problems like in Singapore, where haze phenomenon is a major and recurring problem. Haze levels from uncontrolled Indonesian forest fires hit a highest of PSI 401 in June 2013 [6].
These airborne particulate matter can dissolve in rainwater and cause detrimental effects on human health [7]. Acid deposition increase over the years is caused by large population, rapidly growing economy, and the associated systems of energy consumption and production.
During the pre-depositional phase, there is direct human exposure to acidic substances from ambient air. Coming into contact with acid deposition is very dangerous to humans. Sulphur dioxide and nitrogen oxide have particles that can be inhaled and cause many effects [8]. Short term effects include:
- Breathing difficulties. Usually resolve by itself in a few days.
- Reduced visibility, may lead to increased occurrence of car accidents
- Irritation to eyes, nose, throat. Usually resolve by itself in a few days.
- Affect the heart and lungs such as asthma, chronic obstructive pulmonary disease (COPD), or heart failure
Long term (several years) exposure to air pollution, can have increased risk of certain diseases [8]. High ambient pollution from fine particles (PM2.5, particles smaller than 2.5 micrometres), may increase the risk of:
- Cardiovascular effects (such as heart attacks)
- Reduced lung development
- Development of chronic respiratory diseases (such as asthma) in children
- Increase the risk for heart and lung disorders (like asthma and bronchitis)
References
- National Geographic. (n.d.). Effects of Acid Rain. Retrieved 15 March 2017, from http://www.nationalgeographic.com/environment/global-warming/acid-rain/
- Ophardt, E . Charles. Virtual Chembook Elmhurst College. 2003 .Acid Lake Effect. Retrieved 15 March 2017, from http://chemistry.elmhurst.edu/vchembook/195lakeeffects.html.
- National Geographic. (n.d.). Effects of Acid Rain. Retrieved 27 March 2017, from http://www.nationalgeographic.com/environment/global-warming/acid-rain/
- Georgia State University. (n.d.). Effects of Acid Rain on Trees and Soil. Retrieved http://www2.gsu.edu/~mstnrhx/EnviroBio%20Projects/AcidRain/effects.html
- Ophardt, C.E. (2003). In Virtual Chembook: pH. Retrieved February 22, 2017, from http://chemistry.elmhurst.edu/vchembook/196buildings.html.
- Teamsavetheworld.(n.d.). Retrieved 15 March 2017, from http://teamsavetheworld.weebly.com/acid-rain.html
- BBC. (June 21, 2013). Singapore haze hits record high from Indonesia Fires. Retrieved February 22, 2017, from http://www.bbc.com/news/world-asia-22998592.
- Santa Barbara Country Air Pollution Control District. (n.d.). The Air Quality Index. Retrieved February 22, 2017, from https://www.ourair.org/sbc/the-air-quality-index/
- Hu, G. P., Balasubramanian, R., & Wu, C. D. (2003). Chemical characterization of rainwater at Singapore. Chemosphere, 51(8), 747-755. Retrieved February 22, 2017, from, http://www.sciencedirect.com/science/article/pii/S0045653503000286.
- Health Hub. (January 15, 2015). Impact of Haze on Health. Retrieved February 22, 2017, from https://www.healthhub.sg/live-healthy/327/impact_haze_on_health.