Application Exercise 8: Molecules and Drugs
Q1: In allergy sufferers, histamine causes runny noses, red eyes, and other symptoms. Here is its structural formula.
a) Give the chemicals formula for this compound.
The chemical formula for this structure is C5H9N3.
b) Circle the amine functional groups in histamine.
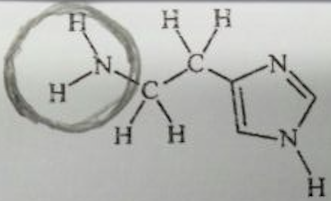
c) Which part (or parts) of the molecule make the compound water-soluble?
The parts of the compound containing nitrogen give this structure its water-soluble property.
- The amine group (NH2).
- The nitrogen to hydrogen single bond bounded at the other end of the structure creates a partial negative dipole and attracts electrons towards itself. It makes this group of atoms electronegative, attracting electron density from the water molecules, making it water-soluble.
Q2: Antihistamines are widely used drugs for treating symptoms of allergies caused by reactions to histamine compounds. This class of drugs competes with histamine, occupying receptor sites on cells normally occupied by histamine. Here is the structure for a particular antihistamine.
a) Give the chemical formula for this compound.
The chemical formula for this compound is C16H21N3.
b) What similarities do you see between this structure and that of histamine (shown in the previous question 1) that would allow the antihistamine to compete with histamine?
The following image shows the structure in Q1.
The following shows the structure of histamine in Q2.
Similarities are a two-carbon chain with a nitrogen at the end (square labelled 1), and a three-atom sequence of N-C-N (square labelled 2). The spatial arrangement of the above 2 features are closely similar as well.
The 5 member ring of histamine and 6 member ring of antihistamine are closely similar as well, allowing the antihistamine to compete.
Histamine contains etyhlamine groups. Antihistamine, similar to histamine, also contain ethylamines groups or similar groups. Such drugs compete with histamine for receptor site and thus block the action of histamine. This makes the drugs anti-histamine, suppressing the symptoms of histamine.
Q3: Consider this statement. “Drugs can be broadly classed into two groups: those that produce a physiological response in the body and those that inhibit the growth of substances that cause infections.” Into which class does each of these drugs fall?
a) Aspirin – Analgesic; physiological response to act directly on the central nervous system and inhibit the prostaglandin and thromboxane synthesis.
b) Morphine – Analgesic; physiological response to act directly on the central nervous system and reduce the sensation of pain.
c) (Keflex) antibiotic – Cephalosporin antibiotic; inhibit infections against many bacteria in the body.
d) Estrogen – Hormonal stimulant; physiological response.
e) Amphetamine – MAO inhibitor; physiological response that stimulates the central nervous system and affects chemicals in the brain and nerves, leading to hyperactivity and impulse control.
f) Penicillin – Antibiotics; inhibit infection especially against many bacterial diseases. 
Q4: Herbal or alternatives medicines are not regulated in the same way as prescription or OTC medicines. In particular, the issues of concern are identification and quantification of the active ingredient, quality control in manufacture, and side effects when the herbal remedy is used in conjunction with another alternative or prescription medicine.
a) What do you think is the evidence from herbal supplement manufacturers that address these issues?

To address the issue of identification and quantification of the active ingredient, quality control in manufacture and side effects, herbal supplement manufacturers should record written procedures to specify critical process steps and factors (eg. extraction time and solvent purity) and acceptance criteria as well as the type of validation to be conducted and the number of process runs.
b) Do you know anything about Singapore’s legislation on the topic?
 Traditional medicinal materials basically fall under two broad categories, mainly those sold in loose or bulk form, and those that are pre-packed for sales (stating information such as product name, brand name, ingredients, dosages and/or instructions for use on the packaging materials).
Traditional medicinal materials basically fall under two broad categories, mainly those sold in loose or bulk form, and those that are pre-packed for sales (stating information such as product name, brand name, ingredients, dosages and/or instructions for use on the packaging materials).
Presently, traditional medicinal materials that are not of finished dosage forms (e.g. capsules, tablets, granules) are not subject to pre-market approval and licensing for their import and sale in Singapore.
However, it is the responsibility of the dealer to ensure that:
- The traditional medicinal materials do not contain any substances controlled under the Poisons Act and other prohibited substances such as Pangamic acid including its salts, Danthron, Suprofen including its salts and Rhodamine B.
- The heavy metal contents of the traditional medicinal materials do not exceed the following limits: Arsenic (5 ppm), Copper (150 ppm), Lead (20 ppm) and Mercury (0.5 ppm).
- The labels and packaging materials of the traditional medicinal materials (if any) do not stipulate any of the 19 diseases/conditions specified in the Schedule of the Medicines (Advertisement and Sale) Act, namely, blindness, cancer, cataract, drug addiction, deafness, diabetes, epilepsy or fits, hypertension, insanity, kidney diseases, leprosy, menstrual disorders, paralysis, tuberculosis, sexual function, infertility, impotency, frigidity, conception and pregnancy
Application Exercise 7: The World of Polymers and Plastics
Q1: When styrofoam packing peanuts are immersed in acetone (the primary component in some nail-polish removers), they dissolve. If the acetone is allowed to evaporate, a solid remains. The solid still consists of styrofoam, but now it is solid and much denser. Explain. Hint: remember that styrofoam is made with foaming agents.
The styrofoam packing polymer is composed of foaming agents. When the acetone dissolves the polymer, the gas from the foaming agent escapes. The polymer, now without any gas in the foaming agent collapses on itself and is denser than before since all the gas has been removed.
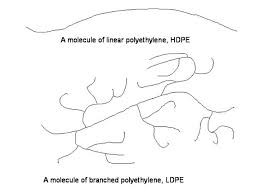
Q2: Consider Spectra, Allied-Signal Corporation’s HDPE fiber, used as liners for surgical gloves. Although the Spectra liner has a very high resistance to being cut, the polymer allows a surgeon to maintain a delicate sense of touch. The interesting thing is that Spectra is linear HDPE, which is usually associated with being rigid and not very flexible.
a) Suggest a reason why branched LDPE cannot be used in this application.
In this application, LDPE, being in a branched arrangement cannot align as closely together as in HDPE and thus it cannot be used in this application to provide the required strength.
b) Offer a molecular level reason for why linear HDPE is successful in this application.
HDPE molecules must be aligned in a specific order and lined up to produce the mechanical strength required. Flexibility also can be produced by using a thin liner of HDPE.
Q3: When you try to stretch a piece of plastic bag, the length of the piece of plastic being pulled increases dramatically and the thickness decreases. Does the same thing happen when you pull on a piece of paper? Why or why not? Explain on a molecular level.
Stretching a piece of plastic narrows the strip from its neck downwards. This is due to the molecules aligning parallel to each other, in the direction of the pull. It changes the 3D structure of the plastic permanently. If the piece of plastic continues to be stretched, the plastic will reach a point when it breaks.
However, when we try to stretch a piece of paper with the same stretching force, the paper becomes torn. This is due to the regularly aligned cellulose molecules in the paper which are hard to alter its arrangement to become stretched.
Q4) A Teflon ear bone, fallow an tube, or heart valve? A Gore-Tex implant for the face or to repair a hernia? Some polymers are biocompatible and now used to replace or repair body parts.
a) List four days properties that would be desirable for polymers used within the human body.

The four properties are non-toxic, unreactive in contact with our body, durable and insolubility when in contact with our body.
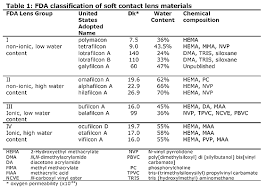
b) Other polymers may be used outside your body, but in close contact with it. For example, no surgeon is needed for you to use your contact lenses-you insert, remove, clean and store them yourself. From which polymers are contact lenses made? What properties are desirable in these materials? Either a call to an optometrist or a search on the Web may provide some answers.
Contact lenses are made from silicone-acrylate, rigid gas permeable polymers (RGP), polymacon(soft lenses) , and other methacrylate. The properties are which are desirable include the insolubility of the lenses in our eyes, non-toxicity, and the ease and comfort of wearing the lenses.
c) What is the difference in the material used in “hard” and “soft” contact lenses? How do the difference in properties affect the ease of wearing contact lenses?
“Hard” contact lenses can refer to the first PMMA lenses invented and rigid gas permeable lenses available. PMMA used to make hard contact lenses reduces the oxygen permeability to the eye. used to make hard contact lenses, can include scleral lenses, while rigid-gas permeable (RGP) lenses can include therapeutic lenses. Due to its regular arrangement of molecules, it allows for better light transmission to provide better optics. However, it is hard and has lower adaptability. RGPs being tough also causes increased prevalence of contact lens related conditions like Giant Papillary Conjunctivitis. Nowadays, it is more commonly used for therapeutic reasons like correction of high astigmatism from keratoconus and to mask any irregularities in refraction from the corneal surface.
Eventually, soft contact lenses replaced them. Soft contact lenses can be made of hydrogel (like Bausch&Lomb Soflens38) or silicone hydrogel (like Coopervision Biofinity). These soft contact lenses provides increased comfort, allowing for a faster contact lens adaption period. More importantly, silicone hydrogel contact lenses, having silicone inside, are more flexible and allows more oxygen pass through it to reach the cornea, preventing corneal hypoxia over long period of wear. Because of this, the soft lenses provides better overall comfort during contact lens wear and is generally preferred over hard contact lenses.
Application Exercise 6: Neutralising the Threat of Acid Rain
Q1: Mammoth Cave National Park in Kentucky is in close proximity to the coal-fired electric utility plants in the Ohio Valley. Noting this, the National Parks Conservation Association (NPCA) reported that this national park had the poorest visibility of any in the country.
a) What is the connection between coal-fired plants and poor visibility?

Sulphur particles are formed from the Sulphur Dioxide that is emitted from the power plants. The sulphur particles do not absorb the sunlight, but reflect it, hence this lowers the visibility of the eastern parks.
b) The NPCA reported “the average rainfall in Mammoth Cave National Park is 10 times more acidic than natural.” From this information and that in your text, estimate the pH of rainfall in the park.

“10 times more acidic” would mean that the pH of the rainfall in the park is one less than the average rainfall. Since average rainfall pH is about 5-6, the pH of the rainfall in the park is 4-5.
Q2: Here are examples of what an individual might do to reduce acid rain. For each, explain the connection to producing acid rain:
a) Hang your laundry to dry it.

Hanging your laundry to dry require less energy as compared to using a dryer. This is because dryer usage requires electricity, which is can be produced through combustion of a coal burning power plant.
b) Walk, bike, or take public transportation to work.

All of these options help to reduce NOx emission released from using a personal vehicle.
Apart from the mentioned “walk, bike or taking public transport” options, some companies even arrange for shuttle bus services for their employees. In recent years, carpooling in Singapore has also become more common, such as through “Uberpool”. Not only does this reduce NOx emission, but it is also a more energy efficient and cost-saving alternative to from using a personal vehicle.
c) Avoid running dishwashers and washing machines with small loads.
Running dishwasher and washing machines with small loads consume same electricity as running it with normal loads. Therefore, it is not energy efficient to do so. Over a long run, this leads to more production of SO2.
d) Add additional insulation on hot water heaters and pipes.

Insulation helps to reduce heat loss on hot water heaters and pipes to the environment. Hence, electricity used for heat the water can be reduced to get the heat you desired.
e) Buy locally grown produce and locally produced food.

Buying locally grown produce and locally produced food does not produced as much NOx compared to imported product, since local product need only short time to transport the products to get to the consumer. This does not require as much energy as transportation over further distances such as through air delivery, from country to country.
3a) Give names and chemical formulas for five acids and five bases.
5 acids: Hydrochloric Acid (HCl), Nitric acid (HNO3), Sulphuric Acid (H2SO4), Phosphoric Acid (H3PO4) and Perchloric Acid (HClO4).
5 bases : Ammonia (NH3), Sodium Hydroxide (NaOH), Magnesium Hydroxide (Mg(OH)2), Calcium hydroxide (Ca(OH)2) and Barium Hydroxide (Ba(OH)2).
The table 4.2 below lists some of the more common acids and bases.

3b) Name three observable properties generally associated with acids and bases.
The three observable properties for acids:
– Sour taste
– Turns blue litmus paper red (as seen from the image on Litmus Paper in Acidic Solution below)
– Corrosive
Three observable properties of bases:
– Bitter taste
– Turns red litmus paper blue (as seen from the image on Litmus Paper in Alkaline Solution below)
– Soapy texture

Q4: The concerns of acid rain vary across the globe. Many countries in North America and Europe have websites dealing with acid rain. Either search to locate one (“Canada, acid rain”) or use these links to websites in Canada, the UK, or Europe. What are the issues in Singapore? Does the acid deposition originate outside or inside the Singapore’s borders?
Acid rain is a global phenomenon that affects people around the world, from North America and Europe, Canada and UK, even right here in Singapore. A news report published in 2009 suggests that the native species in Singapore are in danger due to acid rain. NUS findings showed that this may be due to the acidity of the stream in the Bukit Timah Nature Reserve after torrential rain.

The acidity of water comes from sulphur dioxide and nitrogen oxides in the atmosphere, which are produced from industrial pollutants and lightning. However, air and clouds do not have borders, and the pollution emitted in the neighbouring countries may have affected the acidity of waters in Singapore.
In Singapore, there are reports suggesting that acid rain is lowering the pH of streams, thus causing a decrease in wildlife diversity. However, research and monitoring reports from NEA have stated that there is “no increasing trends in rainfall acidity”. Additionally, studies have shown that animals have evolved and adapted to increasingly acidic environment. Despite that, they are likely still in danger of being wiped out.
Application Exercise 5: Water for Life
Q1: How can you purify your water when you are hiking? Name two or three possibilities. Compare these methods in terms of cost and effectiveness. Are any of these methods similar to those used to purify municipal water supplies? Explain.
| Natural Filtration | Simple Distillation | Mechanical Filters | |
| Diagram |  |
|
 |
| Technique | 1. Use a knife or sharp object to pry a section of the tree bark.
2. Roll the tree bark, creating a funnel shape with a small opening at the end. 3. Use vines to hold them together. 4. Fill the funnel from the bottom with twigs, gravel, sand, charcoal, sand, pebbles. 5. Filter the water. |
1. Hold a cool surface above the pot of boiling water. Boiling the water would kill any harmful microorganisms.
2. Allow the water vapour to condense on the cool surface and be collected into a container. The water vapour condensate collected would be rid of most chemical contaminants. 3. Both these steps provide relatively pure water. However, this method . |
1. Insert one end of the mechanical filter into water.
2. Drink from the other end of the mechanical filter. 3. Water that is drawn up through the straw by suction force passes through hollow fibres that filter water particles down to 0.2micrometer across. 4. Mechanical filters may include carbon filters that filter bacteria and plastic filters that filter sediments. |
| Effectiveness | The filtrate may still contain some fine sedimentation.
Time needed to collect the materials for construction. |
Requires fuel and may cause damage to the environment from the soot and CO release.
Takes a very long time to collect an ample amount for drinking. |
Filters are able to get rid of almost all harmful microorganisms and doesn’t require any use of chemicals.
Also, it provides clean water in the shortest possible time, since filtration is done mechanically. |
| Cost | No cost. | Relatively cheap. | Most Expensive. |
Municipal water purification methods generally uses chemicals and sedimentation to kill microorganisms and remove chemical contaminants. The methods mentioned above are generally more mechanical and doesn’t require use of chemicals and hence aren’t very similar to municipal methods.
Q2: Explain why desalination techniques, despite proven technological effectiveness, are not used more widely to produce potable drinking water.

Desalination involves the processes of distillation and reverse osmosis. Both these techniques require the consumption of energy to remove the salt from seawater. In addition, the extensive technology required for this makes these techniques expensive. Therefore, it is usually more feasible to produce potable drinking water.
Q3: Water quality in a chemical engineering building on campus was continuously monitored because testing indicated water from drinking fountains in the building had dissolved lead levels above those established by NEA.
a) What us the likely major source of the lead in the drinking water?
Major source of lead comes from service pipes that contain corrosion of lead (as seen from the image below).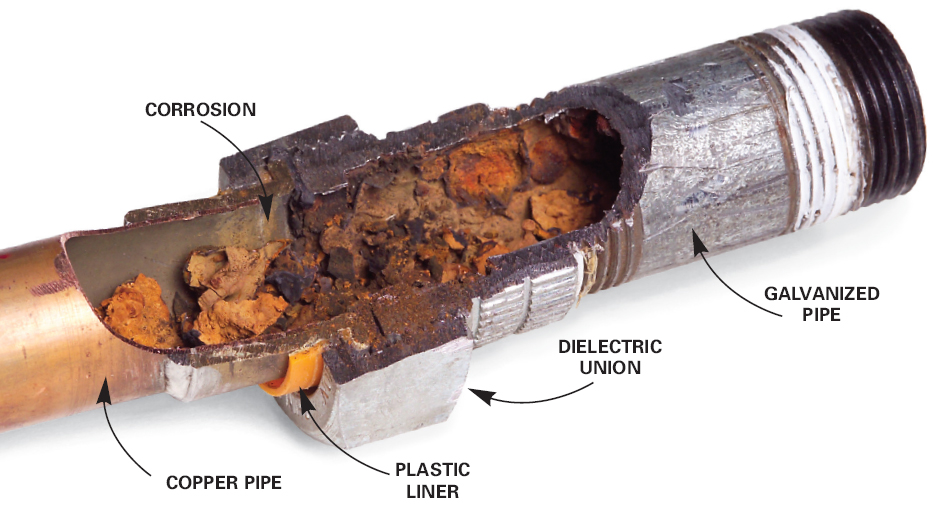
Another problem is when lead solder is used with brass or chrome-plated brass fixtures and faucets. This happens because water has high acidity low mineral content that corrodes pipes and fixture. Even when water has neutral pH, oxygen in water can oxidise the lead and corrosion still happen, especially with hot water. This is significant as it can lead to many harmful health effects such as hearing loss and damage to the nervous system (as shown in the picture below).
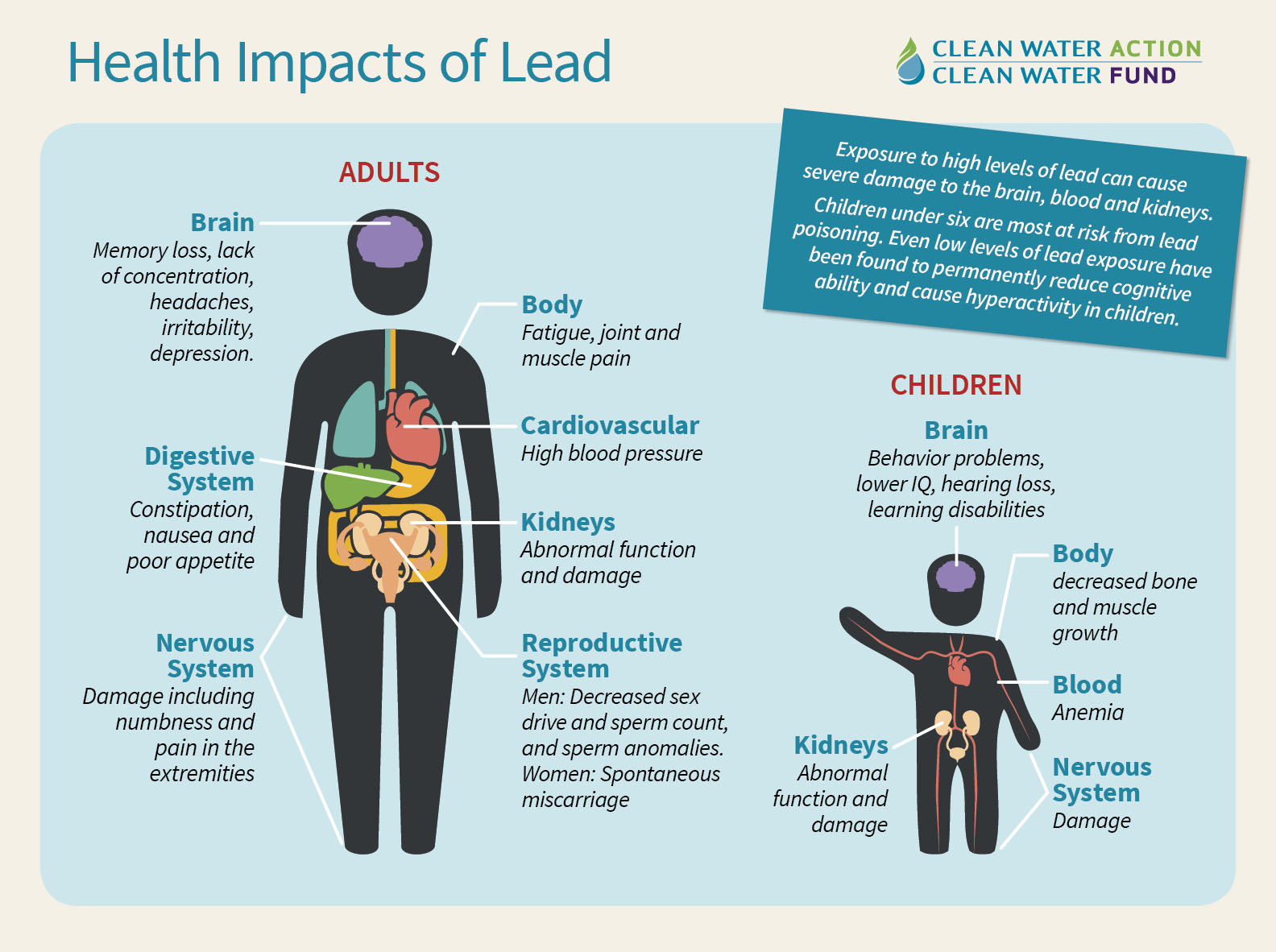
b) Do the research activities carried out in this chemistry building account for the elevated lead levels found in the drinking water? Explain.
No, research activities do not account for elevated lead levels in drinking water. This is because lead is seldom used in research. Even if lead is being used, they can be disposed of properly, giving minimum impact on lead levels in drinking water. Hence, the lead usage in research activities should not result in elevated lead levels in drinking water.
.4) Some vitamins are water-soluble, whereas others are fat-soluble. Would you expect either or both to be polar compounds? Explain.
We have many vitamins in our body. Vitamins B and C are considered water soluble, while the vitamins A,D,E and K are fat soluble.
Fat soluble molecules may have a few polar parts, overall they are non polar. Some fat soluble vitamins are seen from the image below.

Only water-soluble vitamins would be expected to be polar molecules. Some water soluble vitamins are seen from the image below.

Polar covalent bonds in these molecules bond with water molecules through hydrogen bonding and this causes them to dissolve in water. Fat soluble molecules prefer non polar covalent bonds
Application Exercise 4: Global Climate Change
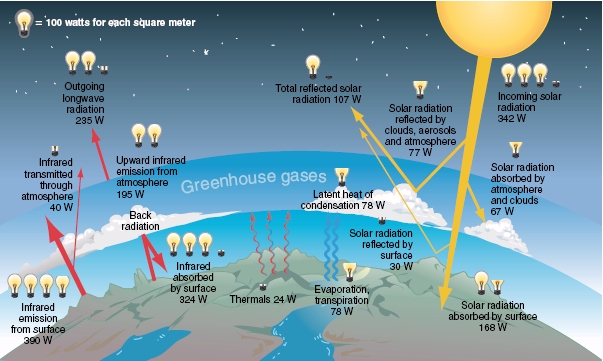
Q1: Understanding Earth’s energy balance is essential to understanding the issue of global warming. For example, the solar energy striking Earth’s surface averages 168 watts per square meter (W/m2), but the energy leaving Earth’s surface averages 390 W/m2. Why isn’t Earth cooling rapidly?
The amount of energy that Earth emitted (390W/m2) is more than twice the amount received (168W/m2). As the atmosphere absorbs quite a bit of the solar energy as heat energy, Earth does not cool rapidly (as seen from the yellow arrows in the picture below).
Q2: Decide and explain where the statement is correct or incorrect. “This winter has lowered my concerns about global warming…”. Explain.
The statement is incorrect because global warming will not cause an immediate effect on the four seasons we have. There is a change in climate due to global warming but not a change in weather.

The difference between climate and weather is that climate is the long term weather of a region, whereas weather is short term. In fact, due to global warming, we will have harsher winters due to the melting of artic ice. Hence, having winters does not improve the long term effects of global warming
Q3: One of the first radar devices developed during World War II used microwave radiation of a specific wave range that triggers the rotation of water molecules. Why was the design not successful?
The image below shows an example of a radar emission device used in World War I.
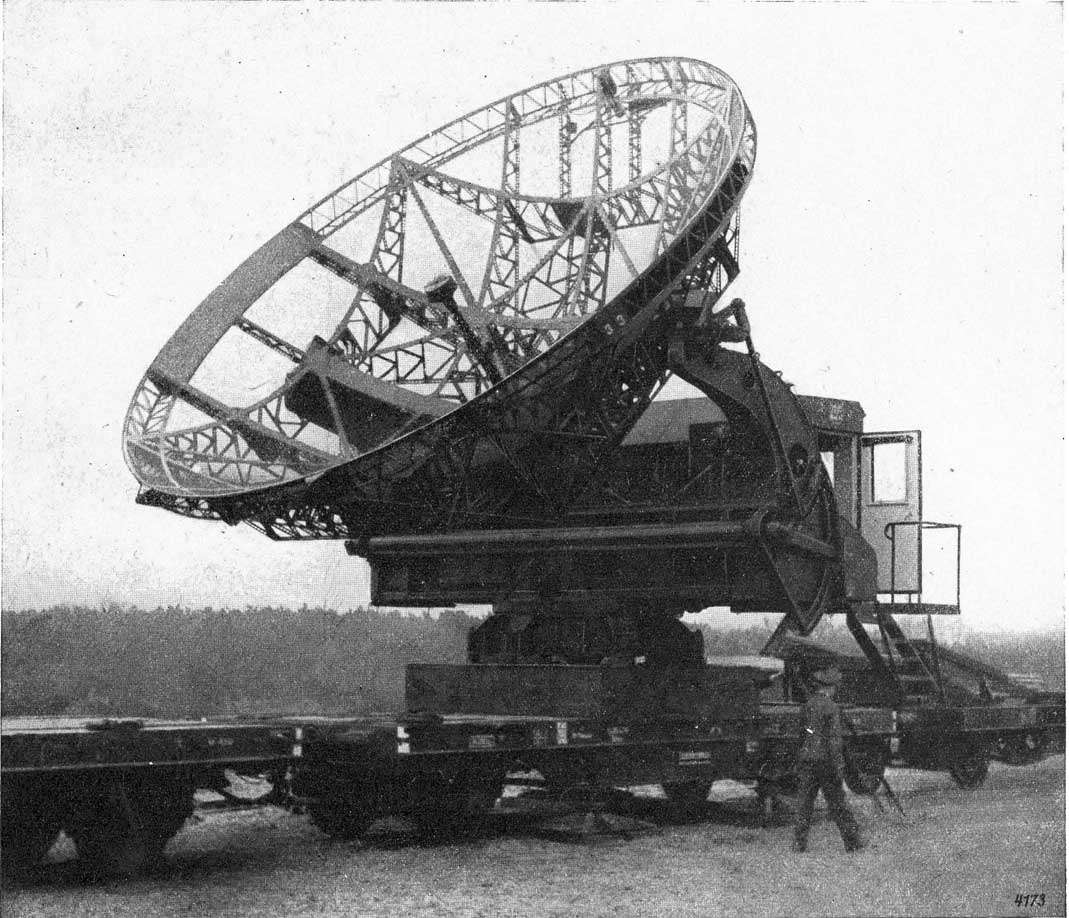
Since the wavelength of radiation used triggers the rotation of water molecules, it means that the radiation can also be absorbed by the atmospheric water vapour. When this radiation is absorbed by water vapour around the radar, it makes the radar unable to detect the target objects and doesn’t reach the target object.
Q4: Now that you have studied air quality (Unit 1), stratospheric ozone depletion (Unit 2), and global warming (Unit 3),which do you believe poses the most serious problem for you in the short run (pick one and explain)? In the long run (pick one and explain)?
While the three problems are quite serious, they might affect us at different time intervals.
For example, air quality is a problem in the short run and can affect us on a daily basis, like the haze problem in Singapore. The stratospheric ozone depletion also affects us but is probably a major factor in the long run as the depletion process is relatively slow.

Lastly, global warming and climate change are influential in the long run as these effects are gradual and usually felt over long periods of time. Some future effects include increase in daily temperature, increased frequency of wamr days and nights, more intense and frequent rainfall, potential increase in wind speed and rise in sea level (as seen from the picture below).

Application Exercise 3: Energy from Combustion
Q1: From personal experience, state whether these processes are endothermic or exothermic. Give a reason for each.
a. A charcoal briquette burns.
This is an exothermic reaction. Heat is released when a charcoal briquette burns.
b. Water evaporates from your skin.
This is an endothermic reaction. When water evaporates from our skin, heat is absorbed, thus our skin feels cooler.
c. Ice melts.
This is an endothermic reaction. Heat is absorbed by the ice from the surrounding, allowing it to melt.
Q2: Chemical explosions are very exothermic reactions. Describe the relative bond strengths in the reactants and products that would make for a good explosion.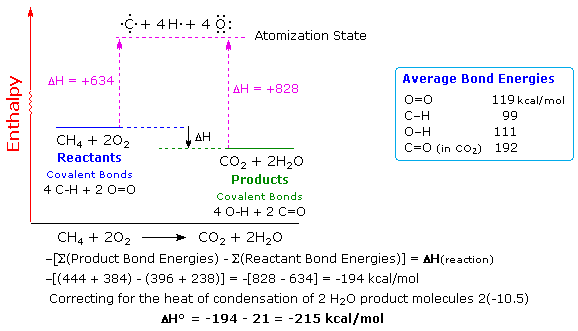
Methane is a type of chemical explosive.
During a methane explosion,CH4 + 2 O2 -> CO2 + 2 H2O.
The bond energies involved are: 4 C-H single bonds (99kcal/mol*4), 2 O=O double bonds (119kcal/mol*2), 2 C=O double bonds (192kcal/mol*2) and 4 O-H single bonds (111kcal/mol*4). The bond energies of the reactants should be lower than the bond energies of the products. The products has a higher bond energy as it releases more energy during bond formation, than give out energy during bond breaking of the reactants. Thus, leading to an exothermic reaction; net negative energy change.
Q3: How might you explain the difference between temperature and heat to a friend? Use some practical, everyday examples.
Heat is the total energy of the molecular movement, while temperature is a measurement of the average thermal energy of the molecule.
A larger beaker of water (beaker A) will have more heat than a smaller beaker of water (beaker B), even though they are of the same temperature. Beaker B has lower energy, so it will take a shorter time to cool down.
It is similar to how a person with more mass has more kinetic energy than a person with less mass, even though they are travelling at the same velocity.
Q4: A premium gasoline available at most stations has an octane rating of 98. What does that tell you about:
a) the knocking characteristics of this gasoline?
The octane rating of gasoline is 98. This means that it has the knocking characteristics similar to a mixture composed of 98% isooctane and 2% n-heptane.
It is categorized as a premium gasoline as it has a higher octane rating than other blends sold at gasoline stations. This means that gasoline is more resistant to knocking than the other blends.
b) whether the fuel contains oxygenates?

The octane rating does not tell us whether the fuel contains oxygenates (such as ethanol). Information on whether the fuel contains oxygenates may be available on other labels found around the pump.