There are various ways in classifying polymers. Firstly is the source of the polymer, whether the polymer is found naturally or synthesized in a lab. Polymers can also be classified via structure, e.g how it is polymerized and the different molecular forces that determine three-dimensional structure of the polymer. Structures of polymers contribute to their properties, which can be very different between individual polymers.
Types of Polymers: Natural polymers can be found in our body as protein and glycogen or in plants as cellulose. Semi-synthetic polymers are neither fully natural nor synthetic, where a natural polymer has been modified in a laboratory. Examples of these modifications are addition of certain functional groups, such as nitrate or acetate, and cleaving a long polymer chain into a shorter chain of desired length
Synthetic polymers are 100% man-made. A common example is polyethene[3] which is the main ingredient in plastic bags. (1, 7)

Properties: Polymers can be elastic, soft or hard. To get these different properties the different chains need to be joined together by various forces to be able to get the appropriate attributes.
Some of the clothes you wear may contain stretch or fabrics that easily dries. Most of them contains tread that is formed from somewhat linear polymer chains, with hydrogen bonding[4] between them which in its form is called fibres. By all the weak interactions between molecules hydrogen bonding[4] is the strongest one, which in large quantities makes fibres very strong, soft and elastic materials. But of course, there is examples of polymers using weaker intermolecular forces as well. One example is elastomers, which is as it sounds – elastic materials. The polymer chains are held together by one of the weakest force, van der Waals interaction. This makes the material elastic and easily stretched due to breaking and formation of new van der Waals forces within the structure.(3, 4)

Thermosetting on the other hand do not have the same reversibility as the thermoplastics. It consists of crosslinked polymers which are heavily branched with many various interactions between each other. Upon heating the crosslink is further stabilized and the material is set and it becomes very hard. This structure is good in that way because it can produce durable and strong materials with a high melting point which prevents it from change. Because of this rigidness, the material cannot be recycled and is therefore not suitable in disposables. (5, 6, 7)

Polymers can be formed in many different ways; mainly condensation and addition polymerisation. Here are some additional methods of formation of polymers:
Formation of Common Polymers
- Condensation Polymerisation
 [9]
[9]
A polymer is formed from the reaction of an acid an an alcohol or an acid and an amine. Both reactions form water as a by-product as ester and amide linkages are formed.
- Addition polymerisation
.jpg)
[10]
A polymer can be formed from the addition of two or more molecules without the formation of any by-product. This can be formed from unsaturated hydrocarbons such as ethene.
Formation of Polymers (addition)
- Free-Radical Polymerisation
[11]
A polymer can be produced from the reaction of two radicals. Radicals are molecules with one unpaired electron; which makes them highly reactive. In this, there are three basic stages: Initiation, Propagation and Termination. The combination of these stages is called chain reaction.
- Cationic Polymerisation
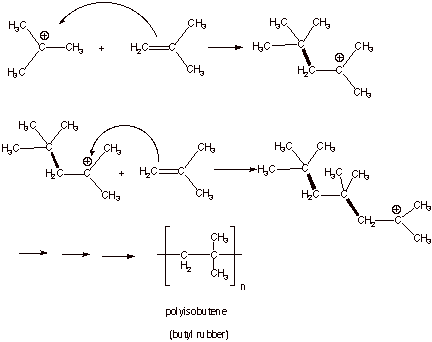
[12]
A polymer can also be produced from the reaction of a cation and an unsaturated carbon bond. Unsaturated carbon bonds refer to carbon-carbon double bonds or triple bonds. Catonic polymerisation also undergoes chain reaction where there are three stages: initiation, propagation and termination.
- Anionic Polymerisation
Anionic Polymerisation is similar to the cationic polymerisation but an anion is used in the reaction rather than a cation.
- Coordination Polymerisation

[13]
Coordination Polymerisation is where a metal complex is used to bind the components of the polymer together. This makes use of the characteristic of the transition metals where it can expand beyond its octet configuration. In the diagram above, ‘M’ represents the Metal whereas ‘L’ represents the components of the polymer, otherwise known as a ligand.
References (1) http://www.examfear.com/free-video-lesson/Class-12/Chemistry/Polymers/part-3.htm (2)http://www.examfear.com/free-video-lesson/Class-12/Chemistry/Polymers/part-4.htm (3)http://www.examfear.com/free-video-lesson/Class-12/Chemistry/Polymers/part-6.htm (4) http://www.modorplastics.com/thermoset-vs-thermoplastics (5) https://plastics.americanchemistry.com/plastics/The-Basics/ (6) http://www.wisegeek.com/what-is-thermoplastic.htm (7) https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry_Textbook_Maps/Map%3A_Organic_Chemistry_(Bruice)/29%3A_Synthetic_Polymers/29.1%3A_There_Are_Two_Major_Classes_of_Synthetic_Polymers (8) http://www.uwosh.edu/faculty_staff/mihalick/materials/Chapter5.pdf (9) http://www.chemistryrules.me.uk/junior/making_polyester.gif (10) http://www.chemhume.co.uk/ASCHEM/Unit%202/Ch9%20Alkanes/poly(ethene).jpg (11) http://www1.biologie.uni-hamburg.de/b-online/library/newton/Chy251_253/Lectures/Free_Radicals/RadicalPolymerization.GIF (12) http://research.cm.utexas.edu/nbauld/polymers.htm (13) https://en.wikipedia.org/wiki/Coordination_polymer