Chemical Characteristic of Greenhouse Gases
Greenhouse gases are capable of trapping heat because they possess the ability to absorb strongly in the infrared spectral band. Such a phenomenon is well illustrated by gas molecules that are made up of three or more atoms (eg: H2O, CO2, CH4). These molecules resonate well in the infrared range of electromagnetic radiation as shown in Figure 1.
Figure 1: Absorption spectrum of common greenhouse gases:
Molecular vibrations caused by the absorption of infrared energy must bring about a change in dipole moment. Such molecules are well adapted to act as infrared absorbers. For instance, Figure 2 shows how CO2 undergo various vibrational motions. Although CO2 does not have a permanent dipole moment as a whole, different vibrational modes can induce a change in dipole moment. The symmetric stretching mode does not result in dipole moment change and therefore is not an infrared active mode. However, CO2 can undergo bending modes that disrupt its molecular symmetry. This results in a dipole moment change that facilitates the absorption of infrared radiation during vibrational processes. Similarly, asymmetric stretching modes will also give rise to absorption of infrared radiation.
Figure 2: Vibrational modes of CO2
Other molecules that possess the property of absorbing thermal infrared radiation through vibrational pathways are H2O, CH4, N2O, O3, and CFCs. As a result, the trapping of long wavelength radiation received from the Sun and thereby contribute to the warming effect in the atmosphere.
These molecules will be analysed in greater detail in the following section.
1. Methane (CH4)
Background
The concentration of methane in the atmosphere has been steadily rising since 1800. Methane’s global warming potential is attributed to its ability to trap incoming solar radiation, thereby enhancing the existing greenhouse effect.
Sources / chemical origin
The pie chart shows the distribution of methane sources.
- Natural sources
Natural sources include wetlands, peat bogs, ocean and termites. Wetlands are areas with waterlogged soils. Water saturation along with warm climate create the conditions needed for production of methane (methanogenesis). Since wetlands are anaerobic, they facilitate fermentation process (conversion of acetate to methane) by microorganisms.H3C-COOH → CH4 + CO2
- Anthropogenic sources
Human activities such as fossil fuel production, agriculture, livestock farming and landfilling constitute major sources of methane.During the extraction of fossil fuels, methane emissions will always be released. This is intensified during the handling, transportation and refinement process, as chemical leakages are common occurrences.
Rice agriculture is also another significant source. Paddy fields are man-made wetlands characterized by high moisture content and oxygen depletion. This creates an ideal environment for methane-producing microbes to decompose organic matter, thereby emitting methane.

Livestock farming is another source of methane. Ruminant animals such as cows, sheep and goats produce large amounts of methane during digestion. Bacteria present in their stomach facilitate fermentation which emits methane as a by-product that is exhaled or released as stomach gas (farts).
Landfilling also produces methane from the decomposition of solid waste. Garbage dumps are full of organic matter such as food waste, newspaper and leaves. Such organic matter gets trapped without oxygen and can be broken down by methane-producing microbes.
Effects & implications
Methane is a greenhouse gas that accounts for 20% of the total radiative forcing. This results in significant heat trapping that worsens global warming and rising sea levels.
2. Carbon Dioxide (CO2)
Background
Carbon Dioxide has various practical applications in modern life including fire extinguishers to control electrical and oil fires where CO2 substitutes water due to its greater mass as compared to air, it can calm the flames. CO2 is also an effective refrigerant, useful when transporting perishable food requires to be chilled.
It is a greenhouse gas as it traps Earth’s heat when it is accumulated in the upper atmosphere and lead to global warming. With concentration of CO2 increasing at about 0.4% per year.
Sources of CO2
- CO2 enters the atmosphere through burning fossil fuels (coal, natural gas, and oil), solid waste, trees and wood products, and as a result of certain chemical reactions (e.g., manufacture of cement)
- Manufacture of Cement, requiring 4.7
 million BTU of energy and generated nearly a ton of CO2
million BTU of energy and generated nearly a ton of CO2
→ CO2 is produced directly when limestone is heated; CO2 is produced indirectly when fossil fuels is burnt to heat the kiln
Calcination (Heating of limestone): CaCO3 –(Heat)→ CaO + CO2
- Combustion of fossil fuels come from various sources, including producing electricity, transportation, industries, etc.
- Clearing the world’s forests has resulted in increasing carbon dioxide levels.
- Trees take in CO2 during photosynthesis but there is now an imbalance of gases in the atmosphere after removing these trees
6 CO2 + H2O -(In the presence of UV light)→ C6H12O5 + 6 O2
- Carbon dioxide is released during respiration and combustion.
- C6H12O5 + 6 O2 → 6 CO2 + H2O
- Eg. Methane
CH4 + 2 O2 → CO2 + 2 H2O
Effects and Implications of CO2
CO2 is one with greatest potential to harm the atmosphere as compared to the rest of the greenhouse gases.
Reason:
- CO2 has the greatest influence and impact having the greatest positive “radiative forcing” (RF) value, hence the greatest average surface warming.
- CO2 stays in the atmosphere for the longest period of time (approximately 800 yeanitrrs from now), as compared to other greenhouse gases such as methane (a decade) which eventually converts into CO2, and nitrous oxide (a century)
Ways for Mitigation
- Switching from burning fossil fuels to alternative fuels such as natural gas, biomass, waste-derived fuels when heating the kiln
- Switching to more fuel-efficient methods to reduce emissions of CO2
- Reduce demand for fuel by improving production process to be more efficient
3. Ozone (O3)

Background
Ozone is typically consists of three oxygen atoms.
It is usually formed when energetic ultraviolet (UV) radiation dissociates molecules of oxygen, O2 to separate oxygen atoms. The free oxygen atoms will recombine to form oxygen molecules but when the free oxygen atom collides with an oxygen molecule it will form ozone shown in the equation below.
O(g) + O2(g) → O3(g)
Due to its chemical structure, ozone is usually unstable because it wants to return to the diatomic stable state. Ozone can be found in the stratosphere or at the troposphere.
Ozone in stratosphere is generally beneficial to human life as it provide a shield by preventing the radiation from the sun by absorbing the ultraviolet light from the sun such as UV-C and UV-B and is produced by photochemical reactions which involve O2.
This ‘shield’ consists of ozone that continuously breaks into oxygen molecules and reform back to ozone molecules. This ‘shield’ is very important to life on earth as scientist believed that without the shield, life on earth will not exist till today.
Ozone in the troposphere is considered to be one of the greenhouse gases and ozone is not wanted in the troposphere. Even though, ozone is scarce in the troposphere with about 0.02 to 0.3 parts per millions it can still do a lot of damage. Ozone in the troposphere is mainly produced from photochemical reactions that involve man-made emissions from industry and automobiles. When carbon dioxide is being released, nitrogen-oxide will also be released which provide the source material for making ozone in the presence of sun. A typical hot and sunny day will be the perfect conditions for ozone production and during evening, when the sun intensity decrease, ozone levels will starts to decease. With increased concentration of ozone levels, it will serve as a key ingredient in smog and as a greenhouse gas.
Effect and Implication of Ozone
Ozone will cause respiration problems to human health, damage vegetation and other common materials. Due to the increase of populations, automobiles and industry, more ozone will be produced in troposphere. When ozone concentration has reach its peak, in severe cases it can damage lung tissues, impair an athlete’s performance, eye irritation, chest pain, coughing, nausea, headaches, chest congestion and results in frequent attacks for those with asthma. In worst case scenario, it can worsen heart disease, bronchitis and emphysema.
Due to over time exposure to ozone, rubber, fibers and paints will be damaged, elastic materials can also become brittle and crack.
4. Nitrogen Oxide (NOx)


Background
Nitrogen Oxide in its most common forms are Nitric Oxide (NO) and Nitrogen Dioxide (NO2). While the Environmental Protection Agency is more focused on preventing the emissions of NO2 in the troposphere, Nitrogen Oxide in the form of Nitrous Oxide (N2O) is a major factor in the depletion of ozone in both the troposphere (i.e., below 10,000 feet above sea level) and in the stratosphere (50,000 – 150,000 feet). Based on the U.S. Environmental Protection Agency, the nitrous oxide (N2O) in 2014 accounted for about 6 percent of all U.S. greenhouse gas emissions from human activities.
Yet, N2O has a Global Warming Potential (GWP) 265 to 298 times that of CO2. Basically, much more efficient at trapping heat compared to carbon dioxide. for a 100-year timescale. N2O emitted today remains in the atmosphere for more than 100 years, on average. When considering that N2O has a long half-life, estimated at from 100 to 150 years, any impact on its concentration due to anthropogenic (man-made) sources will cause a lasting effect for centuries.
Another analysis of N2O, it is the reaction with ozone in the stratospheric layer. The photo-chemistry of N2O is taken from a paper titled “Stratospheric ozone depletion due to nitrous oxide: influences of other gases” by R.W. Portman.
Nitrous oxide in the stratosphere is broken down in the middle stratosphere and above via photolysis:

and reaction with O(1D)

and

The NO produced in the final reaction is the primary source of reactive nitrogen (NOx). N2O is now the largest ozone-destroying gas emitted by human activities based on ODP-weighted emissions.
Sources of NOx
European Union emission inventory report (1990 – 2011) under the UNECE Convention on Long-range Trans boundary Air Pollution (LRTAP)
From the BBC, burning fossil fuels and wood is one source of the increase in atmospheric nitrous oxide, however the main contributor is believed to be the widespread use of nitrogen-base fertilisers. Sewage treatment plants may also be a major source of this gas.
Since the Industrial Revolution, the level of nitrous oxide in the atmosphere has increased by 16%.
5. Chloroflurocarbon (CFCs)
Background
Chlorofluorocarbon (CFC) is an organic compound that contains carbon, chlorine, and fluorine, produced as a volatile derivative of methane and ethane.
CFCs are unreactive, stable and poorly soluble in water. They are also nontoxic, nonflammable.
Sources of CFCs
Anthropogenic sources that includes aerosol sprays, blowing agents for foams and packing materials, solvents and refrigerants. They are widely used for industrial and chemical purposes.

Effects and Implications of CFCs
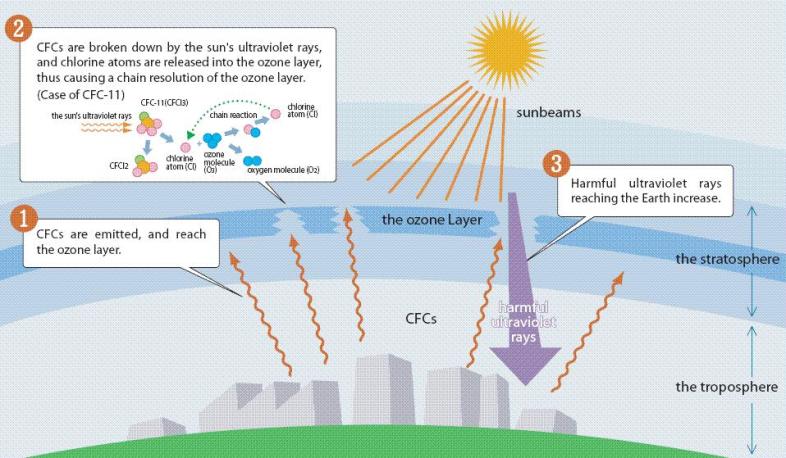
In the stratosphere the CFC molecules are broken down by solar ultraviolet radiation (UV rays) and release chlorine atoms that can react with the ozone molecules, resulting in the depletion of ozone layer.
Cl+O3→ClO+O2(step 1)
ClO+O.→Cl+O2(step 2)
O3+O.→2O2(Overall reaction)
Chlorine acts as a catalyst and initiates the breakdown of ozone by combining with a freed oxygen to produce two oxygen molecules. After each reaction, chlorine begins the destructive cycle again with another ozone molecule. One chlorine atom can thereby destroy thousands of ozone molecules. As ozone molecules are being broken down they are unable to absorb any ultraviolet light and exposure to harmful UV rays is increased.
CFCs have lifetime in the atmosphere of 20 to 100 years and hence one free chlorine atom from a CFC molecule can result in a significant environmental impact, destroying ozone molecules for a long time.
6. Water Vapour (H2O)
Background
Water vapour is the gaseous phase of water. It is one state of water within the hydrosphere. Water vapor is also one of the most important greenhouse gas in the atmosphere. Heat radiated from Earth’s surface is absorbed by water vapor molecules in the lower atmosphere. The water vapor molecules, in turn, radiate heat in all directions. Some of the heat returns to the Earth’’s surface. Thus, water vapor is a second source of warmth (in addition to sunlight) at the Earth’s surface.
Sources of Water Vapour
- Natural Sources of water vapour
(a) Evaporation
Water is found in lakes, oceans, swamps, and soil, as well as in all living creatures and plants. When heat is gained from the sun, through exertion, or by artificial means, the water molecules become excited and spread out. The loss of density is called ‘evaporation’, and results in water rising into the air forming clouds of water vapour.
(b) Transpiration
Transpiration is the process by which moisture is carried through plants from roots to small pores on the underside of leaves, where it changes to vapor and is released to the atmosphere. Research have also found that 10 %of the moisture found in the atmosphere is released by plants through transpiration. The remaining 90% is mainly supplied by evaporation from oceans, seas, and other bodies of water (lakes, rivers, streams).
(c) Volcanic explosion
Large, explosive volcanic eruptions inject water vapour (H2O), carbon dioxide (CO2), sulfur dioxide (SO2), hydrogen chloride (HCl), hydrogen fluoride (HF) and ash (pulverized rock and pumice) into the stratosphere to heights of 10-20 miles above the Earth’s surface. The most significant impacts from these injections come from the conversion of sulfur dioxide to sulfuric acid (H2SO4), which condenses rapidly in the stratosphere to form fine sulfate aerosols. (ugss)
Sulfur dioxide reacts with water to form sulfurous acid.
SO2(g) + H2O(l) → H2SO3(aq)
Substances in the upper atmosphere then catalyze the reaction between sulfurous acid and oxygen to form sulfuric acid.
2H2SO3(aq) + O2(g) → 2H2SO4(aq)
Sulfuric acid thus condenses rapidly in the stratosphere to form fine sulfate aerosols. These increase in aerosols increases the reflection of radiation from the Sun back into space and thus cool the Earth’s lower atmosphere or troposphere; however, they also absorb heat radiated up from the Earth, thereby warming the stratosphere.
2. Anthropogenic source of water vapour
(a) Burning of gasoline and natural gas.
Eg: burning of gasoline (octane) produces both water vapor and carbon dioxide.
2C8H18 (g) + 25O2 (g) → 16CO2 (g) + 18H2O (l)
(b) Burning of natural gas (methane)
CH4(g) + 2O2 (g) → CO2 (g) + 2H2O (l)
Effects and Implications of Water Vapour
As the temperature of the atmosphere rises, more water is evaporated from ground storage (rivers, oceans, reservoirs, soil). Because the air is warmer, the absolute humidity can be higher (in essence, the air is able to ‘hold’ more water when it’s warmer), leading to more water vapour in the atmosphere. As a greenhouse gas, the higher concentration of water vapour is then able to absorb more thermal Infrared radiation (IR) energy radiated from the Earth, thus further warming the atmosphere. The warmer atmosphere can then hold more water vapour and so on and so on. This is referred to as a “positive feedback loop” (Yale, N.a) . Hence, the feedback loop in which water is involved is critically important to projecting future climate change. The research conducted by Andrew Dessler and colleagues from Texas A&M University in College Station managed to conclude that the heat-amplifying effect of water vapour is potent enough to double the climate warming caused by increased levels of carbon dioxide in the atmosphere. This meant that if there is a 1°C change caused by CO2, the water vapour will cause the temperature to go up another 1°C. When other feedback loops are included, the total warming from a potential 1°C change caused by CO2 is, in reality, as much as 3°C. ‘.
References
A/P Kuwata, M. CM4015 Atmospheric Chemistry Lecture notes, 2017.
Scottish Sceptic. (2014). A Scientist’s Guide to Greenhouse Warming. Retrieved 25 March 2017, from http://scottishsceptic.co.uk/2014/11/24/a-scientists-guide-to-greenhouse-warming/
Overview of Greenhouse Gases. (2017, February 14). Retrieved April 03, 2017, from https://www.epa.gov/ghgemissions/overview-greenhouse-gases
Hausfather, Z., & Hausfather, Z. (2008). The water vapor feedback – Yale Climate Connections. Yale Climate Connections. Retrieved 24 March 2017, from http://www.yaleclimateconnections.org/2008/02/common-climate-misconceptions-the-water-vapor-feedback-2/
Greenhouse Gases | Monitoring References | National Centers for Environmental Information (NCEI). Ncdc.noaa.gov. Retrieved 24 March 2017, from https://www.ncdc.noaa.gov/monitoring-references/faq/greenhouse-gases.php
The Water Cycle and Different Stages of The Water Cycle – Conserve Energy Future. Conserve Energy Future. Retrieved 24 March 2017, from http://www.conserve-energy-future.com/water-cycle.php
Kenneth A. McGee, T. (1997). Impact of Volcanic Gases. Pubs.usgs.gov. Retrieved 24 March 2017, from https://pubs.usgs.gov/of/1997/of97-262/of97-262.html
It’s Water Vapor, Not the CO2 – American Chemical Society. American Chemical Society. Retrieved 24 March 2017, from https://www.acs.org/content/acs/en/climatescience/climatesciencenarratives/its-water-vapor-not-the-co2.html
“Carbon Dioxide.” World of Earth Science. . Retrieved March 23, 2017 from Encyclopedia.com: http://www.encyclopedia.com/science/encyclopedias-almanacs-transcripts-and-maps/carbon-dioxide
Greenhouse Effect. (n.d.). Retrieved March 24, 2017, from http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/grnhse.html
Overview of Greenhouse Gases. (2017, February 14). Retrieved March 24, 2017, from https://www.epa.gov/ghgemissions/overview-greenhouse-gases
Emissions from the Cement Industry. (n.d.). Retrieved March 24, 2017, from http://blogs.ei.columbia.edu/2012/05/09/emissions-from-the-cement-industry/
The Link to the Ozone Problem. (n.d.). Retrieved April 03, 2017, from http://earthguide.ucsd.edu/virtualmuseum/climatechange2/10_1.shtml
Ozone in the Troposphere. (n.d.). Retrieved April 03, 2017, from http://www.windows2universe.org/earth/Atmosphere/ozone_tropo.html
Chemical of the Week — Ozone. (n.d.). Retrieved April 03, 2017, from http://scifun.chem.wisc.edu/chemweek/ozone/ozone.html
Weingroff, M. (n.d.). Introduction to Ozone. Retrieved April 03, 2017, from https://www.ucar.edu/learn/1_5_1.htm
Chlorofluorocarbons. (n.d.). Retrieved April 03, 2017, from http://www.encyclopedia.com/environment/encyclopedias-almanacs-transcripts-and-maps/chlorofluorocarbons
L. (2016, July 21). Depletion of the Ozone Layer. Retrieved April 03, 2017, from https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Kinetics/Case_Studies%3A_Kinetics/Depletion_of_the_Ozone_Layer
Chemistry – greenhouse-chloroflourocarbons and the ozone layer (n.d), Dynamicscience.com.au. Retrieved 28 March 2017, from http://www.dynamicscience.com.au/tester/solutions1/chemistry/greenhouse/cfc.htm
Ozone Depletion | Chlorofluorcarbons | CFCs | Stratospheric Ozone (n.d), Ozone-hole.org.uk. Retrieved 28 March 2017, from http://www.ozone-hole.org.uk/05.php
The Ozone Hole. Theozonehole.com (n.d), Retrieved 28 March 2017, from http://www.theozonehole.com/cfc.htm
Benhar.net.technion.ac.il. Retrieved 7 April 2017, from http://benhar.net.technion.ac.il/files/2013/02/NO-molecule.jpg
E. (n.d.). The Carbon Cycle. Retrieved April 07, 2017, from http://eschooltoday.com/ecosystems/the-carbon-cycle.html












