-
How can you purify your water when you are hiking? Name two or three possibilities. What are the relative costs and effectiveness of these alternatives? Are any of the methods similar to those used to purify municipal water supplies? Explain.
Several options exist for purifying water while hiking. The traditional method is to boil it. Boiling kills many microorganisms that may make you sick, but requires time and will not remove chemical contamination. Boiling also requires time, fuel, and may release soot and CO to the environment.
Another method of water purification is with iodine. It is easy and effective in twenty minutes, but iodine should not be used long-term., In addition, pregnant women and people with thyroid conditions should avoid purification with iodine. While iodine renders water bacteriologically safe, it doesn’t remove chemical contaminants. Many people dislike the taste of iodine-treated water.

Lastly, filtering cleans water mechanically. Travel water filters are good microfiltration unit that can remove: “harmful cocci, bacteria, protozoa, fungi, cysts, and parasites are totally removed, including the chemically resistant infectious agents of Giardia, the amoebic and shigella dysenteries, and those causing typhoid, cholera bilharzia and a long list of other dangerous diseases.” Filtration has the advantage over other methods mentioned above as it does not require chemicals. However, filters are the most expensive option out of the rest but a good filter pumps out good water in a few minutes and is reusable.
2. Explain why desalination techniques, despite proven technological effectiveness, are not used more widely to produce potable drinking water.
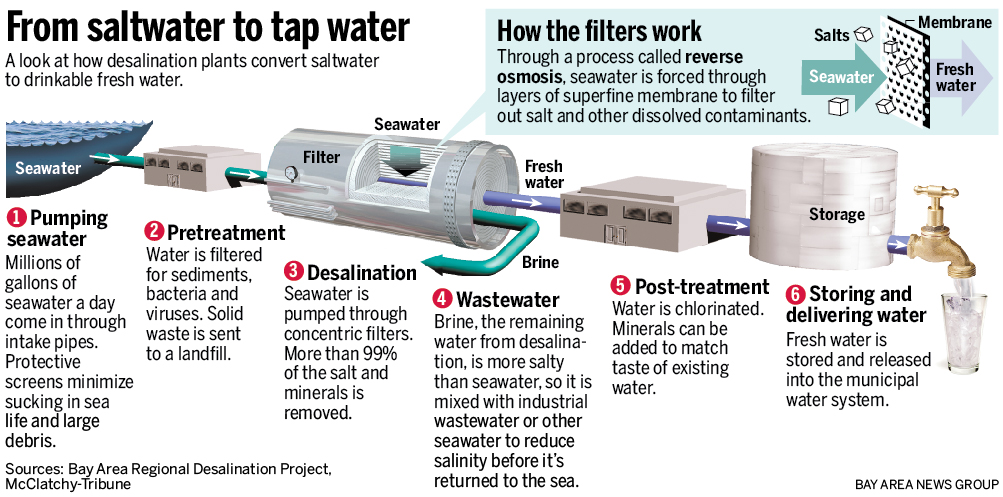
The two most common desalination techniques are distillation and reverse osmosis. Both of these require energy to remove salts from seawater or brackish water, and thus inherently are expensive. If a less expensive option is available (such as hauling fresh water from a distance), then the less expensive option is used.
3. Water quality in a chemical engineering building on campus was continuously monitored because testing indicated water from drinking fountains in the building had dissolved lead levels above those established by NEA.
a. What is the likely major source of the lead in the drinking water?
The likely source of lead is from solder in the pipe joints or from lead pipes themselves.b. Does the chemical research carried out in this chemistry building account for the elevated lead levels found in the drinking water? Why or why not?
Research activities should not contribute to lead in the drinking water, assuming that any lead compounds are disposed of using prescribed methods. Although many undergraduate chemistry experiments used to use lead, most now have been redesigned to avoid it and other toxic metal ions completely.4. Some vitamins are water-soluble, whereas others are fat-soluble. Would you expect either or both to be polar molecules? Explain.
Only water-soluble vitamins would be expected to be polar molecules. Though a fat-soluble vitamin will often have individual polar bonds or small regions of the molecule, overall this is out-weighed by non-polar sections. Polar covalent bonds attract to water through hydrogen bonding and may allow the molecules to dissolve in water, while non-polar covalent bonds favour interactions with the non-polar chains in lipids.
Month: February 2017
Application Exercise 4: Global Climate Change

1. Understanding Earth’s energy balance is essential to understanding the issue of global warming. For example, the solar energy striking Earth’s surface averages 168 watts per square meter (W/m²), but the energy leaving Earth’s surface averages 390 W/m². Why isn’t Earth cooling rapidly?
Even though the amount of energy emitted is more than double the amount received, Earth does not cool rapidly because the atmosphere retains much of the emitted energy.
2. Describe & explain where the stamen is correct or incorrect. Explain.

When temperature measurements are extrapolated into the future, predictions made by Arrhenius of a 5–6 °C rise in the average temperature of the planet’s surface may need to be revised. Current estimates from the United Nations predict that average temperatures will increase somewhere between 1.4 °C and 5.8 °C (2.5 °F and 10.4 °F) by the year 2100. Other scientists, looking at a possible doubling of CO 2 emissions in the future, estimate a temperature increase between 1.0 °C and 3.5 °C (1.8 °F and 6.3 °F). Future temperature changes can be influenced, at least to a considerable extent, by the human beings who inhabit this planet. We are a long way from the out-of-control hothouse of Venus, but we face difficult decisions. These decisions will be better informed with an understanding of the mechanism by which greenhouse gasses interact with radiation to create the greenhouse effect. For that, we must again take a submicroscopic view of matter.
3. One of the first radar devices developed during World War II used microwave radiation of a specific wave range that triggers the rotation of water molecules. Why was the design not successful?
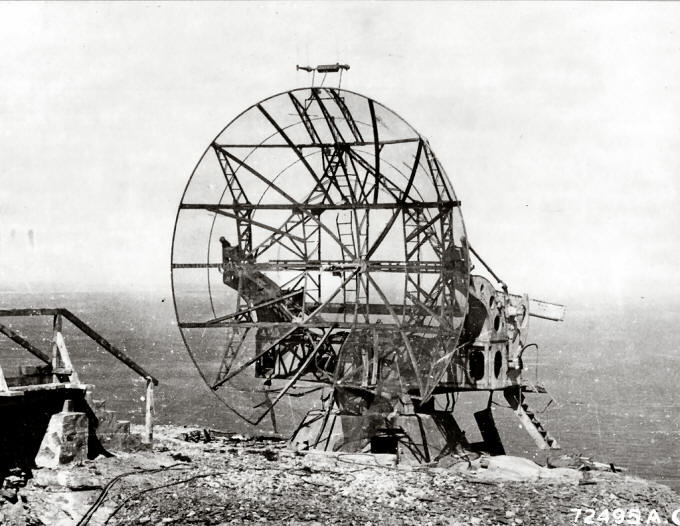
One of the first radar devices developed during World War II used microwave radiation of a wavelength that triggers the rotation of water molecules. This was unfortunate because:
i. it heated up the air around the machine.
ii. it caused diseases in the operators of the radar.
iii. absorption of microwave radiation by water in the atmosphere interferes with the detection of intended objects.
4. Now that you have studied air quality (Unit 1), stratospheric ozone depletion (Unit 2), and global warming (Unit 3), which do you believe poses the most serious problem for you in the short run (pick one and explain)? In the long run (pick one and explain why)?

Answers will likely depend a little on geography. Air quality will likely be the most serious short term concerns of my group, especially with the speed industrial is growing. Take a look at quality of air from Beijing, polluted by the factories or the cost saving method that result in haze and deforestation in Indonesia. The ozone hole is probably too far removed for students like us to have felt the impact directly. Depending on the perceptions of seriousness of climate change, ozone layer depletion will likely be the most serious long term concern we have.
Application Exercise 3: Energy from Combustion

- From personal experience, state whether these processes are endothermic or exothermic. Give a reason for each.
a. A charcoal briquette burns

Exothermic. A charcoal briquette releases heat as it burns.
b. Water evaporates from your skin
Endothermic. Water absorbs the heat necessary for evaporation from your skin, and your skin feels cooler.
c. Ice melts

Endothermic. Ice absorbs the necessary heat to melt from the environment.
- Chemical explosions are very exothermic reactions. Describe the relative bond strengths in the reactants and products that would make a good explosion.

The bond energies involved are: C–H single bonds, 416 kJ/mole; O=O double bonds, 498 kJ/mole; H–O single bonds, 467 kJ/mole; C=O double bonds, 803 kJ/mole. The bond energies of the products are larger than those of the reactants. This will lead to a large negative net energy change indicating an exothermic reaction.
- How might you explain the difference between temperature and heat to a friend? Use some practical, everyday examples?
Wouldn’t you rather spill a drop of hot coffee on you than the whole cupful at the same temperature? Although the drop and the cup full of coffee may initially have the same temperature, you will receive a bigger burn from the bigger volume of coffee because it has the higher heat content. Heat is a form of energy. In contrast, temperature is a measurement that indicates the direction heat will flow. Heat always flows from an object at high temperature to an object at lower temperature. This means that if hot coffee is added to cold coffee, heat will flow from the hot liquid to the cold liquid, and the final temperature of the mixture will be between the original temperatures of the two individual solutions. Heat depends on the temperature and on how much material is present.
- A premium gasoline available at the most stations gas an octane rating of 98. What does that tell you about:

a. The knocking characteristics of this gasoline
Gasoline with an octane rating of 98 has the same knocking characteristics as a mixture composed of 98% isooctane and 2% n-heptane. As a “premium gasoline,” it has a higher octane rating than other blends sold at gasoline stations and hence is more resistant to knocking than these blends.
b. Whether the fuel contains oxygenates
The octane rating provides no information about whether or not the fuel contains oxygenates. Other labels around the pump should reveal this information.
Ozone Layer
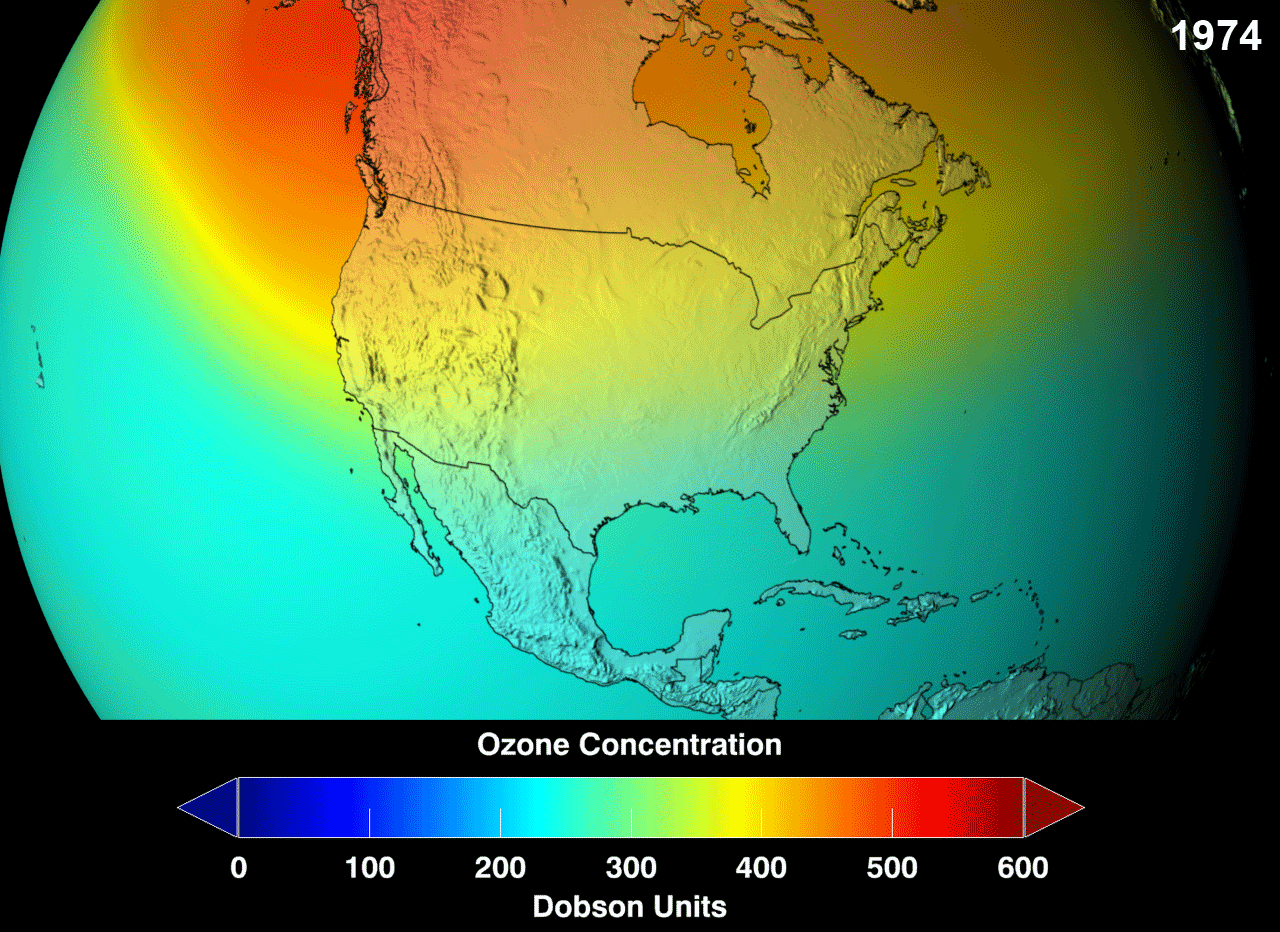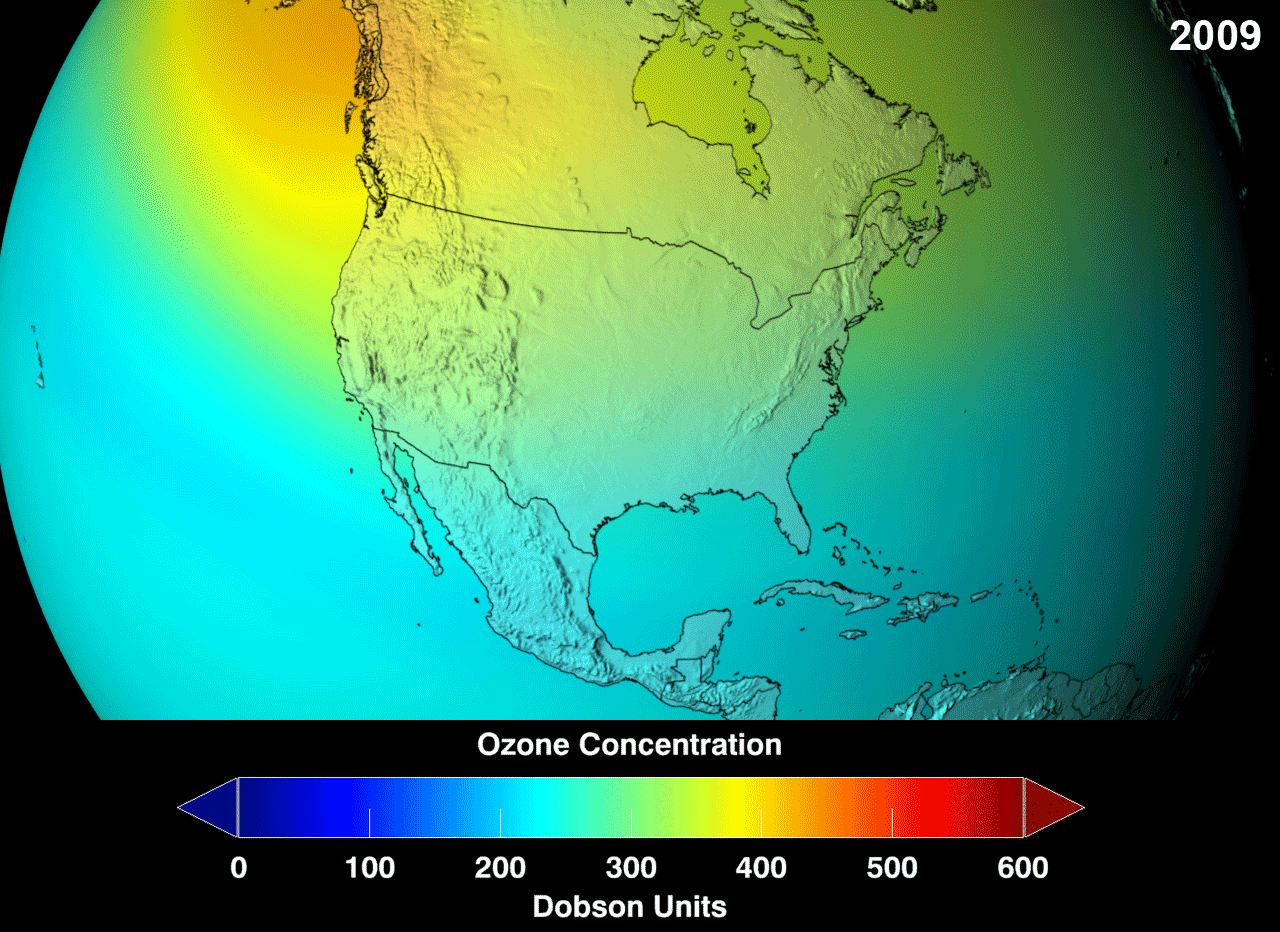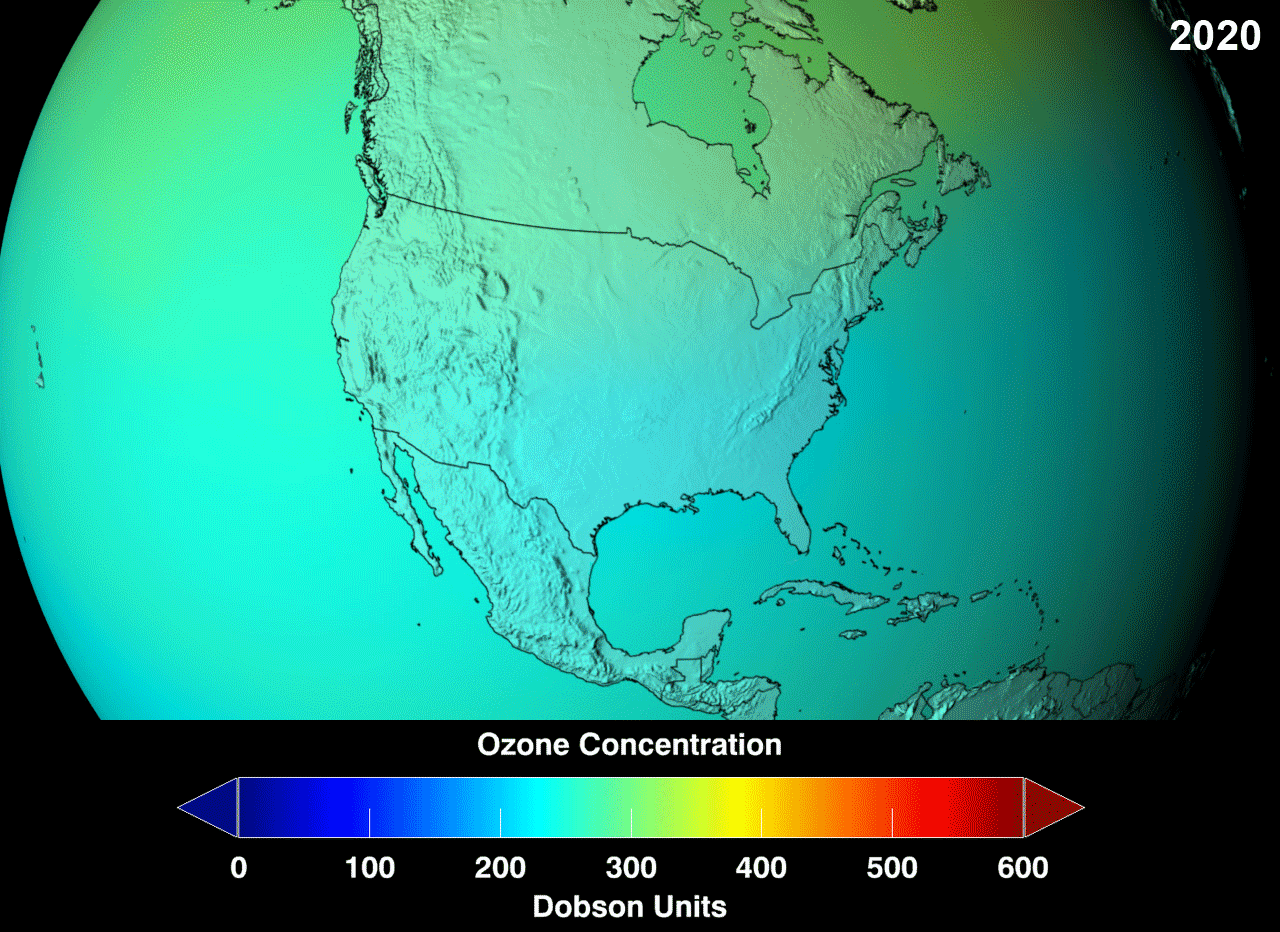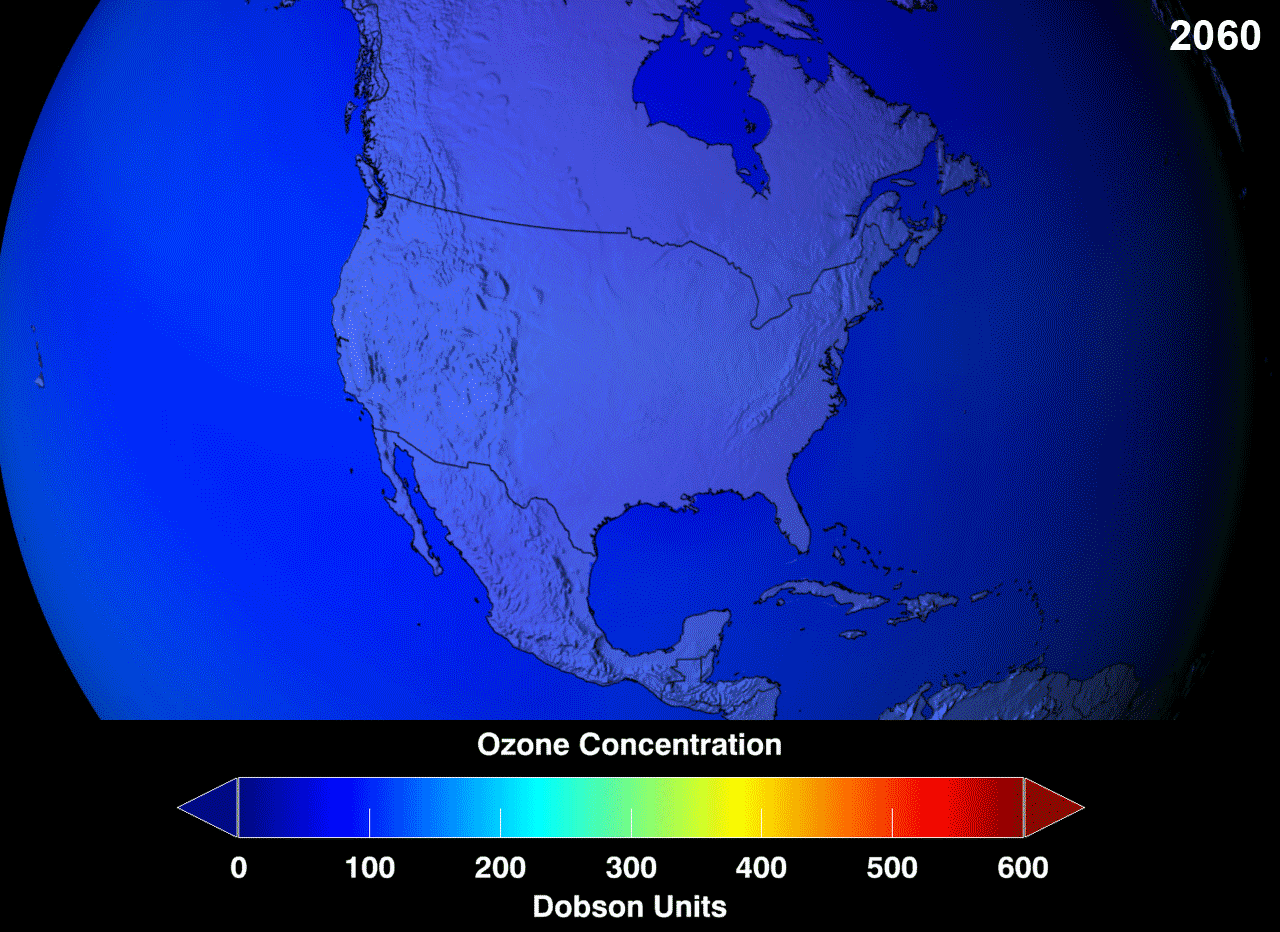
Definition
The ozone layer or ozone shield is a region of Earth’s stratosphere that absorbs most of the Sun’s ultraviolet (UV) radiation. It contains high concentrations of ozone (O3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 parts per million of ozone, while the average ozone concentration in Earth’s atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 20 to 30 kilometres (12 to 19 mi) above Earth, although its thickness varies seasonally and geographically.

The ozone layer absorbs 97 to 99 percent of the Sun’s medium-frequency ultraviolet light (from about 200 nm to 315 nm wavelength), which otherwise would potentially damage exposed life forms near the surface.
Fun Fact: The United Nations General Assembly has designated September 16 as the International Day for the Preservation of the Ozone Layer.
When was the ozone layer discovered?
The ozone layer was discovered in 1913 by the French physicists Charles Fabry and Henri Buisson. Measurements of the sun showed that the radiation sent out from its surface and reaching the ground on Earth is usually consistent with the spectrum of a black body with a temperature in the range of 5,500–6,000 K (5,227 to 5,727 °C), except that there was no radiation below a wavelength of about 310 nm at the ultraviolet end of the spectrum. It was deduced that the missing radiation was being absorbed by something in the atmosphere. Eventually the spectrum of the missing radiation was matched to only one known chemical, ozone. Its properties were explored in detail by the British meteorologist G. M. B. Dobson, who developed a simple spectrophotometer (the Dobsonmeter) that could be used to measure stratospheric ozone from the ground. Between 1928 and 1958, Dobson established a worldwide network of ozone monitoring stations, which continue to operate to this day. The “Dobson unit”, a convenient measure of the amount of ozone overhead, is named in his honor.
Formation of ozone
Ozone present in the stratosphere forms a protective layer, known as ozone layer , ozonosphere or ozone umbrella. Its concentration in atmosphere is about 10 ppm. In the upper atmosphere, atmospheric gases absorbs the sun’s radiation and release molecules . In lower atmosphere , atmospheric oxygen get dissociated and subsequently combines with molecular oxygen of the upper stratosphere, thereby producing ozone.
O2—–(UV radiation)—-> O+O
O2 + O ———–> O3
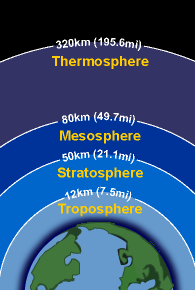
Different layers of the atmosphere
(Adapted from: http://www.conserve-energy-future.com/ozone-layer-and-causes-of-ozone-depletion.php)
To understand ozone layer, it would be helpful to know the different layers of the atmosphere. The earth’s atmosphere is composed of many layers, each playing a significant role. The first layer stretching approximately 10 kilometers upwards from the earth’s surface is known as the troposphere. A lot of human activities such as gas balloons, mountain climbing, and small aircraft flights take place within this region.
The stratosphere is the next layer above the troposphere stretching approximately 15 to 60 kilometers. The ozone layer sits in the lower region of the stratosphere from about 20-30 kilometers above the surface of the earth. The thickness of the ozone layer is about 3 to 5 mm, but it pretty much fluctuates depending on the season and geography.
Ozone layer is a deep layer in earth’s atmosphere that contain ozone which is a naturally occurring molecule containing three oxygen atoms. These ozone molecules form a gaseous layer in the Earth’s upper atmosphere called stratosphere. This lower region of stratosphere containing relatively higher concentration of ozone is called Ozonosphere. The Ozonosphere is found 15-35 km (9 to 22 miles) above the surface of the earth. The thickness of the ozone layer differs as per season and geography. The highest concentrations of ozone occur at altitudes from 26 to 28 km (16 to 17 miles) in the tropics and from 12 to 20 km (7 to 12 miles) towards the poles.
Importance of Ozone
(Adapted from: http://jontymagicman.expertscolumn.com/article/importance-ozone-layer-environment)
The presence of ozone layer in the stratosphere is vital as it absorbs the ultraviolet radiation that is harmful to life and prevent them from reaching the Earth’s atmosphere . If these radiations are allowed to reach the Earth’s atmosphere they will increase the temperature of lower atmosphere to such an extent , that it will be impossible for any life to survive on earth. In addition, these UV radiations can cause severe radiation damage in human and animals such as DNA mutation and skin cancer.
O3 + UV radiations——–> O2 + O
Depletion of Ozone
Mechanism of ozone depletion includes:
Natural process : Ozone in the upper atmosphere absorbs UV radiations of short wavelength and release atomic oxygen. This natural mechanism however do not upset the equilibrium of ozone because atmospheric oxygen absorbs UV radiation of wavelength of shorter than 240nm and photo dissociates into two oxygen atoms. This oxygen combines O2 molecules to form ozone.
Anthropogenic Process: CFCs used in refrigerants and aerosol sprays destroy ozone molecules. At the stratosphere, CFCs breaks to produce chlorine radicals. These chlorine radicals react with ozone molecules and destroy the ozone molecules in a series of reaction:
CFCl3 + UV Light ——- > CFCl2 + Cl
Cl + O3 ———– > ClO + O2
ClO + O ———– > Cl + O2
The free chlorine radical produced will react and destroy another ozone molecule again.
Impacts of CFCs on ozone layer

In 1985, a hole the size of the United States appeared in the ozone layer above the Antarctic. Without the ozone layer, more UV-B rays from the sun penetrate the atmosphere with various inconvenient results, such as a massive increase in skin cancers, reduced crop productivity, depletion of fish stocks, and climate changes resulting in floods and famine.
The scientists rushed to the conclusion, over the next few years, that the depletion of the ozone layer was due to chemicals known as chlorofluorocarbons, or CFCs, which are used in such things as aerosols, hamburger packaging and refrigerators. Most scientists agree that a reduction of 85 per cent in CFC emissions is needed immediately – just to stabilise conditions.
In Montreal, however, the top brains from twenty-four countries decided on a reduction of only 50 per cent, and then not until 1998. Moreover, they were talking only about consumption of CFCs. In fact, they’ve actually agreed to let the big companies increase their production of CFCs.
Negative consequences of ozone depletion
Adapted from: http://www.bcairquality.ca/101/ozone-depletion-impacts.html
Stratospheric ozone filters out most of the sun’s potentially harmful shortwave ultraviolet (UV) radiation. If this ozone becomes depleted, then more UV rays will reach the earth. Exposure to higher amounts of UV radiation could have serious impacts on human beings, animals and plants, such as the following:
Harm to human health

- More skin cancers, sunburns and premature aging of the skin.
- More cataracts, blindness and other eye diseases: UV radiation can damage several parts of the eye, including the lens, cornea, retina and conjunctiva.
- Cataracts (a clouding of the lens) are the major cause of blindness in the world. A sustained 10% thinning of the ozone layer is expected to result in almost two million new cases of cataracts per year, globally (Environment Canada, 1993).
- Weakening of the human immune system (immunosuppression). Early findings suggest that too much UV radiation can suppress the human immune system, which may play a role in the development of skin cancer.
Adverse impacts on agriculture, forestry and natural ecosystems

- Several of the world’s major crop species are particularly vulnerable to increased UV, resulting in reduced growth, photosynthesis and flowering. These species include wheat, rice, barley, oats, corn, soybeans, peas, tomatoes, cucumbers, cauliflower, broccoli and carrots.
- The effect of ozone depletion on the Canadian agricultural sector could be significant.
- Only a few commercially important trees have been tested for UV (UV-B) sensitivity, but early results suggest that plant growth, especially in seedlings, is harmed by more intense UV radiation.
Damage to marine life

- In particular, plankton (tiny organisms in the surface layer of oceans) are threatened by increased UV radiation. Plankton are the first vital step in aquatic food chains.
- Decreases in plankton could disrupt the fresh and saltwater food chains, and lead to a species shift in Canadian waters.
- Loss of biodiversity in our oceans, rivers and lakes could reduce fish yields for commercial and sport fisheries.
Damages to animals

- In domestic animals, UV overexposure may cause eye and skin cancers. Species of marine animals in their developmental stage (e.g. young fish, shrimp larvae and crab larvae) have been threatened in recent years by the increased UV radiation under the Antarctic ozone hole.
Materials

- Wood, plastic, rubber, fabrics and many construction materials are degraded by UV radiation.
- The economic impact of replacing and/or protecting materials could be significant.

