- The Earth does not cool rapidly because the Carbon Dioxide and Water Vapor on the Earth’s surface absorb the heat from the Sun and radiate the energy to keep the Earth warm.
- The statement is incorrect. They do not realise that the extreme weather they are experiencing could in fact be a result of climate change brought about by global warming.
- This is because the absorption of microwave radiation by water vapor in the atmosphere will interferes with the detection of intended objects.
- In the short run, the burning of fossil fuels contributes to global warming and worsen the air quality. Air quality has a direct impact on our everyday lives and can affect human health over a much shorter time frame than processes such as global warming. It is thus more of a concern in the short run.In the long run, global warming will be the most serious problem, as it may cause people who live at the low altitude islands to have no place to live due to the rise in water level cause by the melting of ice in the north and south pole.
Month: February 2017
Application Exercise 3
1a) Exothermic. Combustion gives out heat.
b) Endothermic. Liquid water absorbs heat to become vapour and have more kinetic energy to evaporate away.
c) Endothermic. Solid absorbs heat to become liquid.
2) 
To give an exothermic reaction, bond strengths in products have to be higher than those in reactants. To give a highly exothermic reaction, bond strengths in products should be much higher than those of reactants. Highly negative value should be obtained.
3) Temperature is a measure of the average speed of the molecular motion, and it determines the direction of heat flow. Heat is the energy that flows from a hotter to a colder object. Heat is a consequences of motion at the molecular level.
A glass of boiling water has a high temperature, therefore it is hot. One can feel the heat from the glass due to the transfer of energy from the hot water to your hand. This transfer of energy is referred to as heat.
4a) An octane rating of 98 means that the mixture contains 98% isooctane and 2% n-heptane. It burns smoothly and is more resistant to knocking than octane with lower rating.
b) The octane rating does not show us whether the fuel oxygenates.
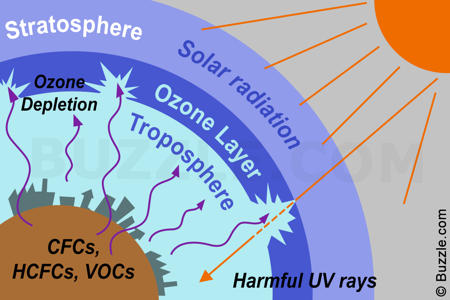
Second lecture: The Ozone Layer
From the group activity, we discussed about the advantages and the disadvantages of the Ozone Screen and the Ozone layer. The ozone screen is more protective and solid whereas the ozone layer is more uniform.
The ozone layer is in a steady state. The rate of the damaging the ozone layer is the same as the rate of forming the ozone layer.