- In allergy sufferers, histamine causes runny noses, red eyes, and other symptoms. Here is its structural formula.
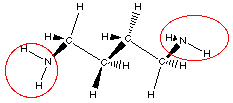 b) Histamine – amine groups
b) Histamine – amine groups
a) Give the chemical formula for this compound.
C5H9N3
b) Circle the amine functional groups in histamine.
(circled in red on diagram)
c) Which part (or parts) of the molecule make the compound water-soluble?
The amine groups contribute to the solubility of the molecule due to the highly electronegative nature of nitrogen atom that makes the molecule polar and so able to interact with the polar water molecules.
2. Antihistamines are widely used drugs for treating symptoms of allergies caused by reactions to histamines compound. This class of drugs competes with histamines, occupying receptor sites on cells normally occupied by histamines.
a) Give the chemical formula for this compound.
C16H21N3
b) What similarities do you see between this structure and that of histamine that would allow for antihistamine to compete with histamine.
Ethylamine moiety (-CH2-CH2-N-)
3. Consider this statement. “Drugs can be broadly classed into 2 groups: those that produce a physiological response in the body and those that inhibit growth of substances that cause infections.”
Drugs that illicit physiological response:
Estrogen, amphetamine, morphine
Drugs that inhibit growth of infection-causing substances:
Penicillin, aspirin, antibiotic (Keflex)
4. Herbal or alternative medicines are not regulated in the same way as prescription or OTC medicines. In particular, the issues of concern are identification and quantification of active ingredient, quality control in manufacture, and side effects when herbal remedy is used in conjunction with another alternative/prescription medicine.
a) What do you think is the evidence from herbal supplement manufacturers that address these issues?
All prescription and non-prescription are regulated in the US by the Food and Drug Administration (FDA). However, herbal and alternative medicines are treated more like special foods, and not considered as drugs. Therefore herbal and alternative medicines will not be put under the same strict safety and effectiveness measurements as prescription or OTC medicines. Prescription or OTC medicines are considered unsafe until proven safe, whereas herbal and alternative medicines are considered safe until proven unsafe. Hence, herbal supplement manufacturers are not required to put their products under clinical trials to find potential risks and interactions with drugs or other substances.
b) Do you know anything about Singapore’s legislation on the topic?
Under the health science authority (HSA) of Singapore, the requirements of drugs in Singapore are as follows:
(a) Traditional medicinal materials do not contain any substances controlled under the Poisons Act and other prohibited substances such as Pangamic acid including its salts, Danthron, Suprofen including its salts and Rhodamine B.
(b) The heavy metal contents of the traditional medicinal materials do not exceed the following limits: Arsenic (5 ppm), Copper (150 ppm), Lead (20 ppm) and Mercury (0.5 ppm).
(c) The labels and packaging materials of the traditional medicinal materials (if any) do not stipulate any of the 19 diseases/conditions specified in the Schedule of the Medicines (Advertisement and Sale) Act, namely, blindness, cancer, cataract, drug addiction, deafness, diabetes, epilepsy or fits, hypertension, insanity, kidney diseases, leprosy, menstrual disorders, paralysis, tuberculosis, sexual function, infertility, impotency, frigidity, conception and pregnancy.