Unit 9- Molecules and Drugs
Question 1
In allergy sufferers, histamine causes runny noses, red eyes, and other symptoms. Here is its structural formula.
a. Give the chemical formula for this compound.
C5H9N3
b. Circle the amine functional groups in histamine
(circled in picture above)
c. Which part (or parts) of the molecule make the compound water-soluble.
The amine groups.
Question 2
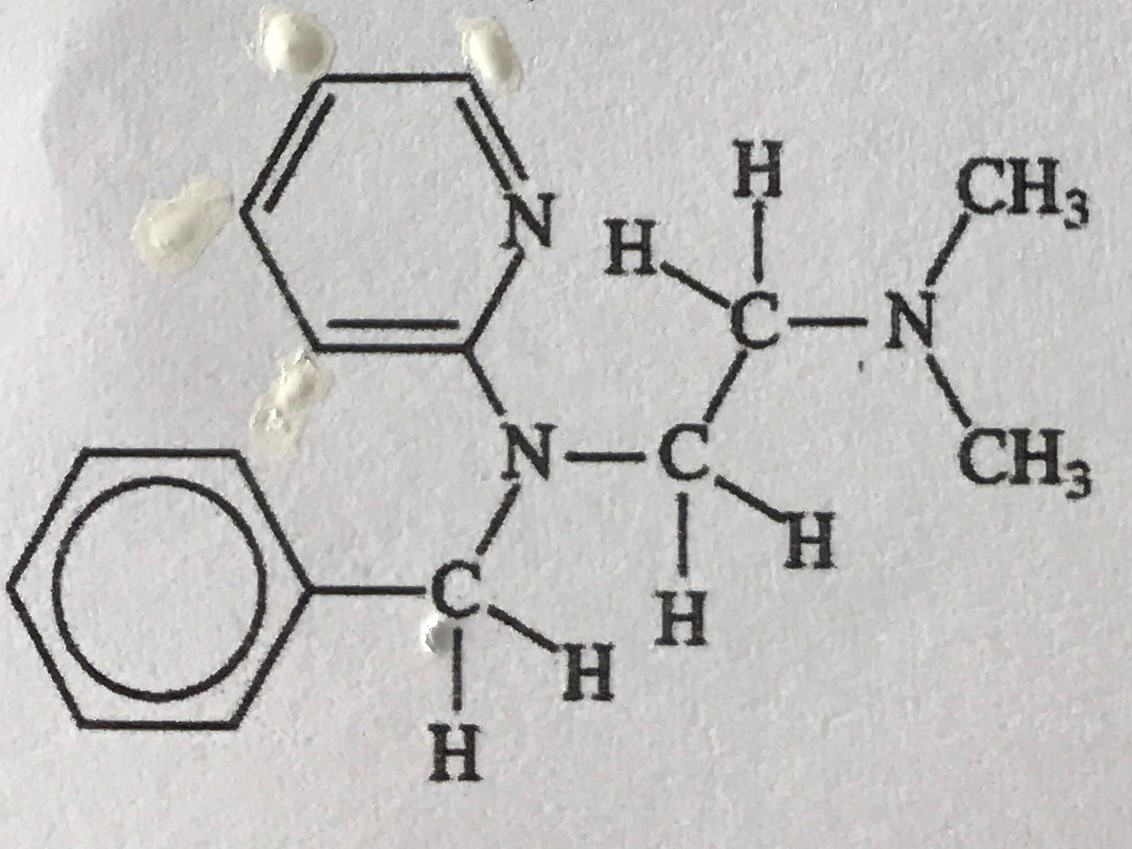
Antihistamines are widely used drugs for treating symptoms of allergies caused by reactions to histamine compounds. This class of drug competes with histamine, occupying receptor sites on cells normally occupied by histamine. Here is the structure for a particular antihistamine.
a. Give the chemical formula for this compound.
C16H21N3
b. What similarities do you see between this structure and that of histamine (shown in the previous question 1) that would allow the antihistamine to compete with histamine?
Like histamine, antihistamine has ringed structures, the terminal N group of histamine is also similar to the amine group of histamine, moreover, in both molecules, the two aforementioned groups are connected by a spacer (akyl chain). These similarities allow antihistamine to compete with histamine and therefore inhibit histamine competitively.
Question 3
Consider this statement. “Drugs can be broadly classed into two groups: those that produce a physiological response in the body and those that inhibit the growth of substances that cause infections.” Into which class does each of these drugs fall?
a.Aspirin, b.Morphine, c.(Keflex) antibiotic, d.Estrogen, e.Amphetamine, f.Penicillin
Produce a physiological response in the body: aspirin, morphine, estrogen, amphetamine (a, b, d, e)
Inhibit the growth of substances that cause infections: (Keflex) antibiotic, penicillin (c, f)
Question 4
Herbal or alternative medicines are not regulated in the same way as prescription or OTC medicines. In particular, the issues of concern are identification and quantification of the active ingredient, quality control in manufacture, and side effects when the herbal remedy is used in conjunction with another alternative or prescription medicine.
a. What do you think is the evidence from herbal supplement manufacturers that address these issues?
Manufacturers usually address these issues by printing a disclaimer/warning on the box/packaging that informs consumers of possible consequences of consuming the product in conjunction with other forms of medication/supplements. This is usually followed by a line advising consumers not to do so.
b. Do you know anything about Singapore’s legislation on the topic?
Traditional medicines that are not in finished dosage forms such as pills or tablets are not subject to pre-market approval and licensing for their import and sale.
However, the salesperson must ensure that the medicine does not contain any prohibited substances.