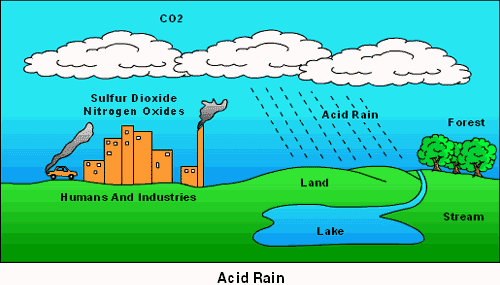
1a) Coal-fired plants produce sulfur dioxide which will lead to the formation of sulfate particles in the air. Sulfate particles in the air can absorb and scatter light, causing poor visibility in Mammoth Cove National Park

b) pH of rainfall in the park is 4.6.
2a) Hanging laundry to dry instead of using dryer will reduce the usage of electricity. Renewable energy sources can better accommodate to the reduced usage of electricity by households and will depend lesser on the burning of fossil fuels for energy. The decrease in dependency of burning fossil fuels for energy will reduce the amount of sulfur dioxides and nitrogen oxides being produced. Formation of acid rain will be reduced due to the low concentration of sulfur dioxide and nitrogen oxides present in the air.

b) Taking public transport, bike or walk can reduce the number of cars present on the road. The combustion of fuels in car will produce nitrogen oxides and by reducing the number of cars on the road, lesser amount of nitrogen oxides will be emitted into the air. This will then reduce the formation of acid rain.


c) Avoid running dishwashers and washing machines with small loads as they do not maximise the use of energy and will lead to the over-consumption of energy. Energy is required to allow the constant flow of water through the tap and electricity is being used to allow washing machines to function. By maximising the use of energy, lesser energy will be wasted and will reduce the consumption of energy. Dependency on burning fossil fuels for energy will be reduced and lesser amount of sulfur dioxides and nitrogen oxides will be produced. This will then lead to the reduction of the formation of acid rain.


d) The installation of additional insulation on hot water heaters and pipes will help to reduce the temperature within the household. As the temperature within the household is being lowered, people within the household will rely lesser on electrical appliances such as fans and aircons to cool their surroundings down. Energy consumption will be reduced and production of energy through burning of fossil fuels will be reduced too, resulting in lesser amount of sulfur dioxides and nitrogen oxides being formed. This will then lead to the reduce the formation of acid rain.
e) Supporting locally grown produce and locally produced food will lead to the reduction in reliance of imported food and products. Importation of food and products from other countries or regions require transportation such as ships and airplanes, which will lead to the production of nitrogen oxides. Reducing the consumption of imported food and products will lead to the reduce in the need of transportation for the imported food and products. This will reduce the production of nitrogen oxides and then the formation of acid rain.


3a) Acids: Hydrochloric acid = HCL, Nitric acid = HNO3, Sulfuric acid = H2SO4, Phosphoric acid = H3PO4, Acetic acid = CH3COOH
Bases: Sodium hydroxide = NaOH, Calcium carbonate = CaCO3, Magnesium hydroxide = Mg(OH)2, Ammonium hydroxide = NH4OH, Calcium hydroxide = Ca(OH)2
b) Acids can generate electric current, turn blue litmus paper red and taste sour. Bases can generate electric current, turn red litmus paper blue and taste bitter.
4) Acid deposition can originate both inside and outside of the Singapore’s border.
The presence of power and industrial plants in Singapore can contribute to acid deposition by producing Sulfur dioxide and Nitrogen oxides as waste products. The heavy reliance on transportations in Singapore can also lead to the increase in production of Nitrogen oxides, causing acid deposition.
There are also other factors that can be found outside of Singapore that can cause acid deposition within Singapore. One example would be the annual haze produced by Indonesia’s slash and burn method. The haze was blown into Singapore by the South-Western wind from Sumatra and contains many air pollutants that may result in acid deposition.
In conclusion, we believe that acid deposition can originate from both inside and outside of the Singapore’s border.