Chemical Concept
How does Reverse Osmosis work?
As explained in the Introduction section, osmosis is a nature-driven process and since reverse osmosis is the converse of osmosis, we can understand that ‘something’ must be initiated in order for reverse osmosis to happen. That ‘something’ is pressure, typically applied by a high pressure pump, to drive water of high salt concentration through the semi-permeable membrane. Recall that in osmosis, water of low salt concentration flows through the membrane and into the region of high salt concentration. The comparison can be illustrated as follows:

The amount of pressure applied in a reverse osmosis system varies, the greater the concentration of salt/contaminants, higher pressure is needed. The importance of the semi-permeable is the fact that it blocks almost 95%-99% of dissolved salts/contaminants from entering the region of clean water. The trapped contaminants are then flushed through as a ‘reject stream’ which allows the membrane to be reusable.
This is known as cross filtration rather than standard filtration where the contaminants are collected within the filter media. The solution crosses the filter, with two outlets: the filtered water goes one way and the contaminated water goes another way. To avoid the build up of contaminants, cross flow filtration allows water to sweep away contaminant build up and also allow enough turbulence to keep the membrane surface clean.[1]
Thus, a reverse osmosis system consists of the following:
- High pressure pump
- Input: Saline/contaminated water feed
- Output: Clean water
- Semi-permeable membrane
- Reject stream (the contaminants)
Shown below is a simple schematic diagram of the reverse osmosis system:

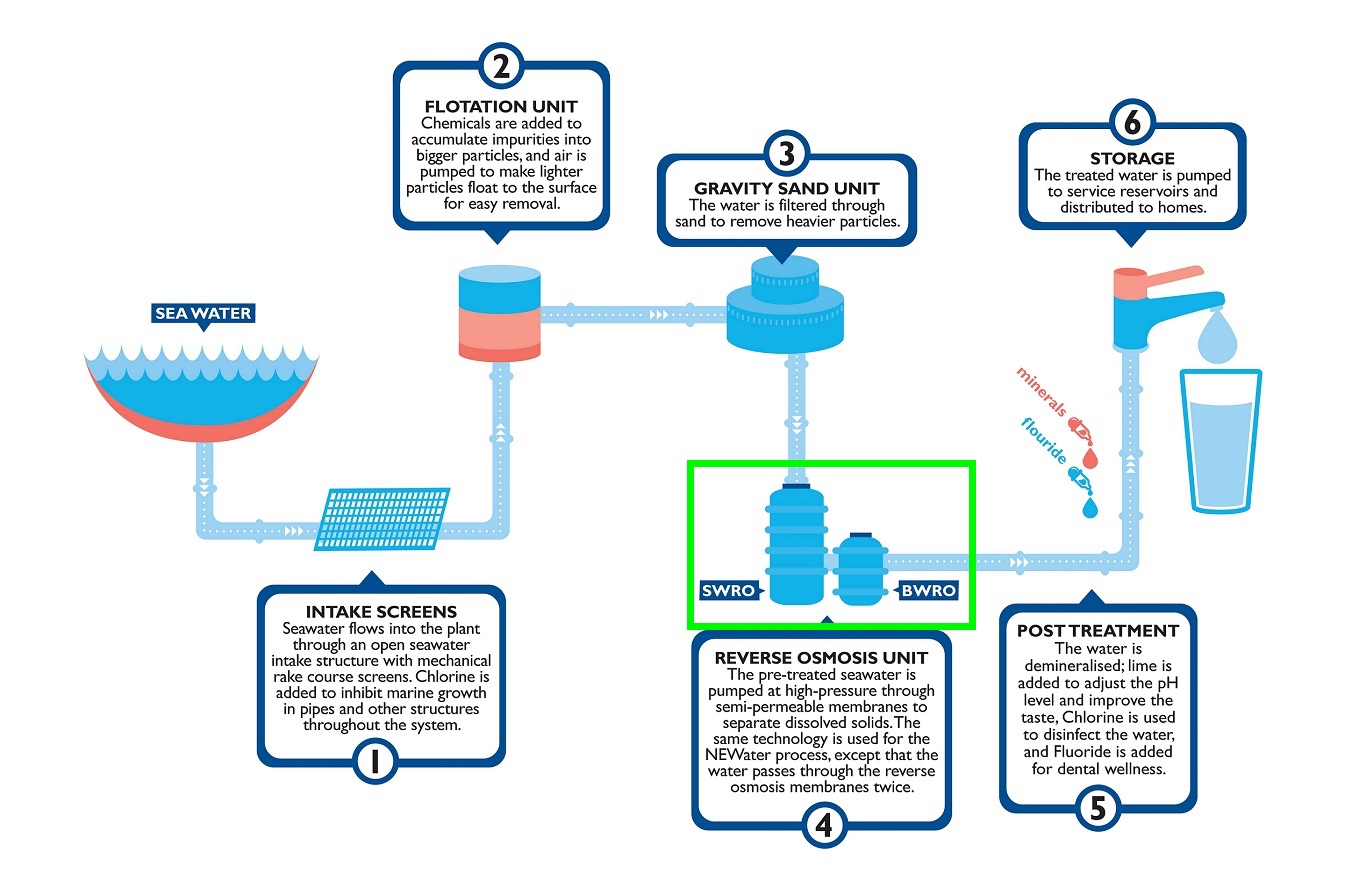
Below is the current application for Reverse Osmosis in Singapore:
What can RO remove?
Now comes the question, what type of water feed can be treated by a reverse osmosis system? As the semi-permeable membrane blocks contaminants based on the size and charge, a reverse osmosis system is capable of removing particles, colloids, organics, bacteria and almost 99% of dissolved salts (ions) from a water feed [2] The illustration below explains why the membrane used in reverse osmosis systems are capable of such removal.

[Retrieved from: http://www.achawater.com/membrane-water-purification.html]
The pores of the membrane are just enough for pure water molecules to pass through trapping anything bigger than 0.0005 micron. In the Implication to Society section, the applications and importance of water treatment via reverse osmosis are discussed.
Also, in the RO system, the greater the ionic charge of the contaminant, the more likely it will be unable to pass through the RO membrane. For example, a sodium ion has only one charge (monovalent) and is not rejected by the RO membrane as well as calcium for example, which has two charges. This explains why an RO system does not remove gases such as CO2 very well as they are not highly ionised in solution and have a very low molecular weight. Therefore, the permeate water can have a slightly lower than normal pH level depending on CO2 levels in the feed water as the CO2 is converted to carbonic acid.[1]
Reverse Osmosis is very effective in treating brackish, surface and ground water for both large and small flows applications. [1]
References
[1] http://puretecwater.com/reverse-osmosis/what-is-reverse-osmosis
[2]Puretec Water (N.D) Reverse Osmosis Systems. Retrieved from http://puretecwater.com/reverse-osmosis/reverse-osmosis-systems