Introduction
Gasoline is a fuel that is distilled from the crude oil. Gasoline consists of organic compounds which exists in the form of various structures and include linear and even cyclic structures such as aromatic or alkane cyclic hydrocarbons. While the composition of gasoline may vary, a common gasoline structure is provided below. In the diagram provided, gasoline is depicted to have 8 carbon atoms and 8 hydrogen atoms.The density of gasoline ranges from 0.71 to 0.77kg/m3.
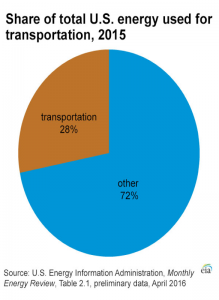
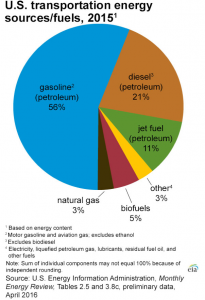
Gasoline plays an important role in our world today. In the United States of America alone, there is a consumption of 140.43 billion gallons of gasoline in the year of 2015. Gasoline is mainly used to fuel internal combustion for engines of cars, motorcycles, light trucks, boats and other transport vehicle. 28% of the total energy consumption in the United States of America is due to the transportation. Out of that 28% of total energy consumption for transportation, gasoline contributes up to 56%, making gasoline the most commonly used transportation fuel in the U.S.A.
This blog covers the chemistry concept of gasoline which includes a brief introduction of gasoline and concepts of gasoline in great details. The concepts which will be covered is as shown below:
1) The combustion of gasoline
2) Production of gasoline
3) Ignition of car engine and preignition
4) Knocking and octane rating
5) Ways to improve octane rating
We will also be discussing the implications of gasoline on our environment.
Chemical Concept of Gasoline
In this section, we will be discussing 5 chemical concepts of gasoline.
Concept 1: Combustion of Gasoline
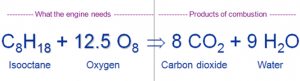
Gasoline is not a simple hydrocarbon when compared to less complexed hydrocarbons such as methane with four hydrogen atoms and one carbon atom. This may cause complications due to incomplete combustion of gasoline. Under complete combustion of a simple hydrocarbon with oxygen, the hydrogen atoms combine with oxygen to form water (H2O) while the carbon atoms form carbon dioxide (CO2) with oxygen. The equation for a complete combustion of gasoline is provided below.
Under incomplete combustion, for example, when a limited supply of oxygen is present, carbon monoxide (CO) and carbon (C) may be produced instead. A general equation for incomplete combustion of a hydrocarbon is provided below:
hydrocarbon + oxygen → carbon monoxide + carbon + water
The production of gasoline involves a process known as fractional distillation and this phenomena is dependent on the differing boiling points of different hydrocarbons. This process is further discussed in the paragraph below.
Concept 2: Production of gasoline:
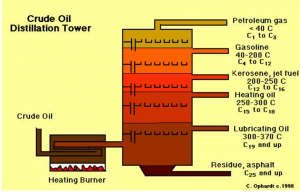
The production of gasoline begins with the fractional distillation of crude oil. The process of fractional distillation separates the crude oil into different components based on the different boiling points of each component. Hydrocarbons with longer carbon chains will have higher boiling points and hence, will remain at the bottom of the distillation column as compared to hydrocarbons with shorter carbon chains. Gasoline has an average of 4-12 Carbon atoms have a relatively low boiling point of 40 – 200 degrees celsius as compared to other components of crude oil. Hence, upon heating the crude oil such that it reaches the boiling point of gasoline, gasoline changes into gaseous form. When this gasoline in gaseous form cools below its boiling point, it condenses into liquids and subsequently, drawn off the distilling column. This process of fractional distillation of crude oil to obtain gasoline can be illustrated using the picture above.
Concept 3: Ignition of Car Engine and Preignition
In order to ignite the car engine, gasoline vapour and air are first drawn into a cylinder. As the both components are drawn into the cylinder, they will be compressed by a piston. The compression will eventually lead to the ignition by spark. Normal combustion occurs as the fuel-air mixture is ignited by the spark plug. The flame produced will travel across the combustion chamber immediately and smoothly until the fuel is used up.
Nevertheless, premature firing known as pre-ignition might also take place as the car engine is ignited. Pre-ignition takes place as compression leads to fuel ignition before the spark occurs. Due to preignition, this condition will lead to lower energy efficiency and higher fuel consumption. The reason pre-ignition happens is the piston is not in its optimal location as the combusted gas expands.
Concept 4: Knocking and Octane Rating
Knocking takes place as violent and unregulated pressure that are several times larger than usual pressure builds up in the engines. Knocking normally takes place after the fuel is ignited by the spark, causing the uncombusted mixture of fuel and air to combust at supersonic speed with an abnormal rise in pressure.As the result, knocking generates an irritating metallic sound. Besides, knocking also results in the loss of power, overheating and eventually leads to engine damage as knocking becomes more serious.
In the last century, the chemical composition was identified to be one of the factors leading to knocking.As the consequence, octane rating was applied to designate the gasoline’s resistance against knocking. Isooctane is assigned an octane rating of 100 as smooth ignition of engine takes place as isooctane is used as the fuel. On the other hand, heptane is assigned an octane rating of 0 as severe knocking of engine occurs when heptane is used as the fuel.
Each particular kind of gasoline is assigned to an octane number which is positive in value. For certain type of gasoline, the octane rating might exceed 100! For an instance, if the gasoline has an octane rating of 95, this illustrates that the gasoline possesses the same knocking characteristics as a mixture of 95% isooctane and 5% heptane.
Concept 5: Ways to increase Octane Rating
Octane rating is defined as a fuel’s ability to resist ‘knock’ and hence a the higher the octane rating, the greater the fuel is in resisting ‘knock’. As knocking reduces energy efficiency and causes serious damage to the vehicle engine, hence there is vigorous research to improve octane rating. Hitherto, there are a few methods implemented or used to be applicable in order to improve the octane rating:
- “Re-form” of Hydrocarbon
Comparing a linear hydrocarbon and a more branched hydrocarbon, the latter has a more compact structure. As the result, this compact structure imparts a more controllable combustion.” Re-forming” of hydrocarbon from a linear hydrocarbon to a more compact structure can be done by passing the linear hydrocarbon over rare and expensive metal catalyst, such as platinum, palladium and rhodium.
- Addition of Anti-knocking Agent
In the previous century, teraethyllead (TEL) used to be added into the gasoline as the anti-knocking agent. Anti-knocking agent helps to reduce knocking besides acting as a lubricant on the pistons. However, through a research done in the late 1970s, it was found out that lead emission from the car exhaust fumes had adverse effects on the IQ of the children living in the cities. The presence of lead also indicates that catalytic converter cannot be used to cut down noxious emission. Following the findings, most of the developed countries have come to a decision to ban TEL due to the harm caused by TEL.
- Addition of more aromatics into the gasoline pool
Due to the addition of more aromatics into the gasoline pool, this as the result, increase the crude oil requirement for every litre of fuel. In addition, aromatics are generally carcinogenic and toxic. The addition of more aromatics into the gasoline also implies that the public’s exposure towards toxic aromatics are also increased. However, as the quantity of aromatics added is minute, hence, this measure is not viewed as a significant problem. This method is implemented in the Europe countries.
- Use of Oxygenated Gasoline
Fuels with oxygen-containing additives are known as oxygenated gasoline The examples of oxygen-containing additives includes ethanol and methyl tertiary-butyl ether (MTBE). As these additives contain oxygen, the oxygenated gasolines combust more completely, thus producing less carbon monoxide as compared to their non-oxygenated counterparts. The details related to the oxygen-containing additives are shown below:
- Ethanol
The addition of ethanol to the gasoline pool is a common practice in countries such as Brazil.
- b) MTBE
Among all measures mentioned to increase octane number, the gasoline added with MTBE has the highest octane rating. Addition of MTBE in the gasoline pool is done commonly in the USA. However, concerns have been raised recently about the addition of MTBE into the gasoline pool as MTBE appears to be leaking into the drinking water in parts of the USA, notably California. The use of MTBE as the fuel additive is being phased out in certain states.
Implications to Society
Due to widespread uses of gasoline in our transportation network, it has caused many implications to our society such as: Environmental Impacts, Toxicity, Flammability
Environmental Impact
According to US Energy Information Administration, a gallon of gasoline combusts to produce 8.74 kilograms of carbon dioxide. Given that 26 percent of 2014 greenhouse gas emissions in the US comes from transportation, and 90% of the fuel is petroleum based including gasoline and diesel, combustion of gasoline is a major source of anthropogenic carbon dioxide, which exacerbates climate change.
Combustion of gasoline in engines also requires high temperature and pressure, resulting in the production of nitrogen oxides, contributing to the production of photochemical smog. The use of leaded petroleum in the past has also resulted in the decrease in efficacy of the catalytic converter, resulting in a greater production of nitrous oxides. During 1962, the “Great Smog” that enveloped London resulted in 4000 more people dying in that week as compared to that period of time during the previous year.
Toxicity
Use of leaded gasoline has resulted in an increase in ambient lead concentration, resulting in higher lead concentration in the blood through inhalation, ingestion of contaminated dust, water and food. Lead accumulated in the body have profound effects on children, affecting development of brain and nervous system. Adults are not spared the harmful effects of lead either, resulting in heightened risk of high blood pressure and kidney damage.
Even with the phasing out of leaded gasoline, unleaded gasoline also contains hazardous chemicals which are present in different compositions. National Institute for Occupational Safety and Health has classified gasoline as a carcinogen. Gasoline contains benzene, xylene, toluene and its additives are ethanol, methanol and methyl tertiary butyl ether. These chemicals are moderately to mildly toxic at acute doses. These toxins are not normally absorbed into our body system, but due to the volatility of the chemicals we can be exposed through breathing in the toxins, or ingesting them.
Flammability
Gasoline, like other hydrocarbons, are flammable and when mixed in air, can rapidly combust and burn. Hence any leakage of gasoline, due to its volatile nature, is a fire hazard which has to be handled with particular care when transporting and storing the liquid. Hence petrol stations often have their gasoline pump equipped with anti-spillage nozzles to prevent spillage, and erect signs to warn people not to expose any naked flame in the station.
Citations:
Innes, J. L. (2000). Biomass Burning and Climate: An Introduction. Advances in Global Change Research Biomass Burning and Its Inter-Relationships with the Climate System, 1-13. doi:10.1007/0-306-47959-1_1
BBC – GCSE Bitesize: Combustion. (n.d.). Retrieved March 22, 2017, from http://www.bbc.co.uk/schools/gcsebitesize/science/ocr_gateway/carbon_chemistry/carbon_fuelsrev2.shtml
U.S. Energy Information Administration – EIA – Independent Statistics and Analysis. (n.d.). Retrieved March 22, 2017, from https://www.eia.gov/tools/faqs/faq.php?id=307&t=11
Colbeck, I & Mackenzie, AR 1994, Air pollution by photochemical oxidants, Air Quality Monographs Vol 1, Elsevier, Netherlands.
Tracton, S. (2012, December 20). The killer London smog event of December, 1952. Retrieved March 22, 2017, from https://www.washingtonpost.com/blogs/capital-weather-gang/post/the-killer-london-smog-event-of-december-1952-a-reminder-of-deadly-smog-events-in-us/2012/12/19/452c66bc-498e-11e2-b6f0-e851e741d196_blog.html?utm_term=.c375c69463df
Lead poisoning and health. (n.d.). Retrieved March 22, 2017, from http://www.who.int/mediacentre/factsheets/fs379/en/
Reese, E., & Kimbrough, R. D. (1993). Acute toxicity of gasoline and some additives. Environmental Health Perspectives, 101(Suppl 6), 115-131. doi:10.1289/ehp.93101s6115
Gasoline. (n.d.). Retrieved March 22, 2017, from http://www.chemistryexplained.com/Fe-Ge/Gasoline.html
Refining Oil. (n.d.). Retrieved March 22, 2017, from http://chemistry.elmhurst.edu/vchembook/513refining.html
Brain, M. (2002, February 06). How Gasoline Works. Retrieved March 22, 2017, from http://science.howstuffworks.com/gasoline1.htm
U.S. Energy Information Administration – EIA – Independent Statistics and Analysis. (n.d.). Retrieved March 22, 2017, from https://www.eia.gov/tools/faqs/faq.cfm?id=23&t=10
Energy Use for Transportation. (n.d.). Retrieved March 22, 2017, from https://www.eia.gov/energyexplained/?page=us_energy_transportation
Gasoline – Energy Explained, Your Guide To Understanding Energy – Energy Information Administration. (n.d.). Retrieved March 22, 2017, from https://www.eia.gov/energyexplained/index.cfm?page=gasoline_home
FirmGreen Fuels Facts. (n.d.). Retrieved March 22, 2017, from http://www.firmgreen.com/fuel/fuel_facts.htm
Lancaster, M. (2002). GREEN CHEMISTRY: An Introductory text. Cambridge, UK: The Royal Society of Chemistry.
Middlecamp, C., Mury, M., Anderson, K., Bentley, A., Cann, M., Ellis, J., & Roberts, K. P. ( 2015). Chemistry in Context: Applying Chemistry to Society (8th Edition ed.). New york: McGraw-Hill Education.
Meeting’s Log:
1st Meeting
Date: 8th March 2017 (Wednesday)
Time: 1430-1600
Duration : 1 hour 30 minutes
Location: Level B1, the Hive, Nanyang Technological University
Participants: Ng Pei Rou, Ang Shihe, Marie Hong Hui Min, Ernest Lim Jun Wei, Maharah Binte Abdul Mahid
Topic Discussed:
- Job allocation of the blog post is discussed.
- The chapter that is assigned is Chapter 4- Energy From Combustion. From this chapter, the topic that is selected is The Chemistry of Gasoline.
Actions To Be Taken:
- Ernest Lim will be in charge of the introduction.
- Marie, Pei Rou and Maharah will be in charge of the chemical concepts.
- Shihe will be in charge of the implication
- For the next meeting, refinement and synergy of content will be done online via Skype and Google Docs.
- Next meeting: 18th March 2017 (Saturday), at 2115-2315
2nd meeting
Date: 18th March 2017 (Saturday)
Time: 2115 – 2315
Duration: 2 hours
Location: Skype
Participants: Ng Pei Rou, Ang Shihe, Marie Hong Hui Min, Ernest Lim Jun Wei, Maharah Binte Abdul Mahid
Topics discussed:
- Refinement and synergy of blog post content.
- Reviewing of the blog post is done by commenting in the Google Docs.
Actions to be taken:
- Second review of the blog post will be done on 22nd March 2017 (Wednesday), after the CM8001 lecture.
Review of The Actions Taken During last Minute:
- All the allocated tasks are completed.
3rd meeting
Date: 22nd March 2017 (Wednesday)
Time: 1500 – 1630
Duration: 3 hours
Location: Lecture Theatre 23, Nanyang Technological University
Participants: Ng Pei Rou, Ang Shihe, Marie Hong Hui Min, Ernest Lim Jun Wei, Maharah Binte Abdul Mahid
Topics discussed:
- Review for the second time of the blog post on Google Docs.
- Job allocation for two video assignments is discussed.
Actions to be taken:
- Video 1, which explains the chemical concepts is allocated to Pei Rou, Marie and Maharah.
- Video 2, which explain the impact, is allocated to Ernest and Shihe.
4th meeting
Date: 29th March 2017 (Wednesday)
Time: 1330 – 1730
Duration: 4 hours
Location: Outside LT20
Participants: Ng Pei Rou, Ang Shi He, Marie Hong Hui Min, Ernest Lim Jun Wei, Maharah Binte Abdul Mahid
Topics discussed:
- Review of both video contents.
- Recording and editing of both videos.
- Submission of blog post and two videos.
Review of The Actions Taken During Last Minute:
- All the allocated tasks are completed.