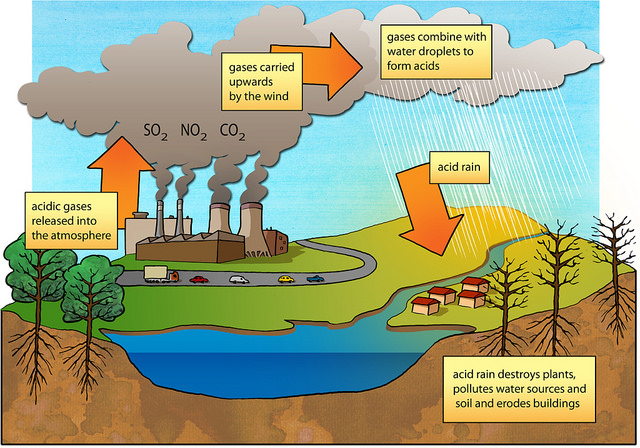
Question 1 (a)
Coal-fired plants release sulphur dioxide, creating sulphate particles that are responsible for60 to 85 percent of the poor visibility in eastern parks.
Question 1 (b)
Normal rain has a pH range of 5–6, so if the rainfall in this park is 10 times more acidic, the pH range must be 4–5.
Question 2 (a)
By hanging outside, you are not using the dryer. As using dryer requires energy consumption, by not using it, we are reducing the production of electricity.
Question 2 (b)
By using the public transport, more people share the energy and fuel consumption as compared to them having their own cars.
Question 2 (c)
Avoid using the machine multiple times as small loads can be reduced with bigger loads, reducing energy consumption.
Question 2(d)
Preventing heat and cold from escaping, reducing energy use.
Question 2 (e)
By buying locally produced produce, there is no need to ship the goods around with ships, trucks, planes, which are huge contributors of SO2 and NO2 that causes acid rain.
Question 3
| Acids | Bases |
| Hydrochloric acid (HCL) | Sodium Hydroxide (NaOH) |
| (Sulphuric acid (H2SO4) | Potassium Hydroxide (KOH) |
| Nitric acid (HNO3) | Ammonium Hyrdoxide (NH4OH) |
| Carbonic Acid (H2CO3) | Magnesium Hydroxide (Ma(OH)2) |
| Phosphoric acid(H2PO4) | Calcium Hydroxide (Ca(OH)2) |
| Turn blue litmus paper pink
Tastes sour Conduct electric current |
Turn red litmus paper blue
Tastes bitter Slippery feel in water |
Question 4
Native species may be wiped out in Bukit Timah Nature Reserve. The acidity of the streams in the nature reserve is more acidic as compared to 20 years ago. The population of certain animal species have decreased and crabs have developed harder shells.
Acid deposition originates both inside and outside of Singapore. Within Singapore, industrialisation and presence of aerosols cause acid deposition. Transboundary sources include forest fires from Indonesia. Forest fires produce carbon dioxide and sulphur dioxide, which combines with rain to make carbonic acid and sulphuric acid.