1a) Burning coal produces some amount of SO2, which reacts with oxygen and water in the air to form sulfuric acid. Molecules of sulfuric acid in the air form tiny droplets (aerosols) that reflects sunlight, thus reducing visibility (much like haze).
1b) Since pH is a logarithmic scale, 10 times increase in acidity translates to 1 point decrease in pH. pH of rainwater is around 5.3 naturally, hence the pH of rainwater in the park is estimated to be around 4.3.
2a) Electricity is needed to run electric dryer and burning of coals will be required to generate electricity. This process emits gases like sulfur dioxide. Sulfur dioxide going into the atmosphere will react with water to form acid rain. Hang clothes to dry will not need electricity.
2b) Large amount of NOx is emitted from vehicles, hence, contributing to acid rain when it reacts with water. By taking public transport, walking and bike will reduce the amount of NOx emission.
2c) Electricity is needed to run the dishwashers and washing machines. By accumulating more dishes, it reduces the usage of the machines, hence, less electricity is used which in term reduces the amount of sulfur dioxide produced from coal-fired electric power plant.
2d) Heating up the water requires burning of fossil fuels. By adding additional insulation, it reduces the amount of heat loss, keeping the temperature slightly higher compared to uninsulated pipes. Hence, less energy is required to heat up the water since temperature can be maintained. Less sulfur dioxide produces from power plants will reduce acid rain.
2e) By buying home grown food, this reduces the need to import food from other countries. This reduces the distance for the food to be transported especially if it is from other countries. Hence, less pollutants like CO2, SO2 and NOx will be produced during transportation and they are sources for formation of acid rain.
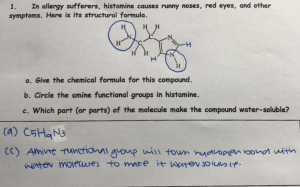
3a) Give the names and chemical formulas for five acids and bases
Hydrochloric Acid, HCl
Sulfuric Acid, H2SO4
Ethanoic Acid, CH3COOH
Phosphoric Acid, H3PO4
Fluoroantimonic Acid, HSbF6
Sodium Hydroxide, NaOH
Ammoniun Hydroxide, NH4OH
Barium Hydroxide, Ba(OH)2
Aluminium Hydroxide, Al(OH)3
Iron II Hydroxide, Fe2(OH)2
3b) Name three observable properties generally associated with acids and bases
Acids generally have a sour taste whereas bases generally taste bitter.
When red litmus paper is dipped into a base it turns red and when a blue litmus paper is dipped into an acid it turns blue
Bases have a soapy feel to it whilst strong acids can burn our hands due to their corrosiveness.
4) The acid depositions can be originated from inside and outside singapore’s borders, but mainly inside.
Singapore does actually experience acid rain to a certain extent. The lower pH record in some location suggest that acidic ions released are contributed by SOx and NOx from coal-burning from heavy industries, in places like Jurong Island and western part.
The acid deposition is also came from ship that used fuel with a much lower sulfur content and emits a lot of SOx.
While outside Singapore’s border, action from our neighbors such as indonesia whose forest caught fire will lead to contributing acid deposition. Other than that, other asian nation which also grow into industrialization can also contribute to large number of SOx emission.
Hence, the acid deposition here originates from both inside and outside Singapore’s borders.