From personal experience, state whether these processes are endothermic or exothermic.
Give a reason for each.
- A charcoal briquette burns.
- Water evaporates from your skin.
- Ice melts.
Answer:
- Exothermic. A charcoal briquette releases heat as it burns.
- Endothermic. Evaporation requires thermal energy from the surrounding. In this sense, thermal energy from your body helps the water on the skin to evaporate. When water evaporates, it takes away thermal energy from the surface of your skin, and your skin feels cooler.
- Endothermic. The molecules in a solid ice are held by strong intermolecular bonds. For the solid ice to melt, these bonds have to be broken. Since energy is needed to break the intermolecular bonds, ice absorbs the necessary thermal energy to do work to break the intermolecular bonds between the molecules of the solid ice. Once the intermolecular bonds are broken, the molecules can now move out of their fixed position and the ice has melted.
Chemical explosions are very exothermic reactions. Describe the relative bond strengths in the reactants and products that would make for a good explosion.
Answer:
Consider a natural gas (methane) explosion:
CH4 + 2 O2 → CO2 + 2 H2O
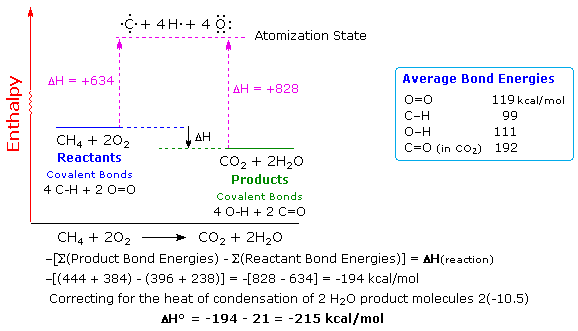
From the above Figure, it should be noted that the bond energies of the products are larger than those of the reactants. This will lead to a large negative net energy change indicating an exothermic reaction.
How might you explain the difference between temperature and heat to a friend? Use some practical, everyday examples.
Answer:
Heat is a form of energy – it is the amount of thermal energy that flows from a hotter region to a colder region. Per contra, the temperature is a measurement that determines the direction of heat flow. The final temperature will be between the original temperatures of the two. Heat depends on the temperature and on how much material is present.
Consider the following experimentation:
Prepare three small basins of water labelled A, B and C as shown below. Place your right hand into basin A and your left hand into basin C. Your right hand would feel cold while your left hand would feel warm.
Remove both hands, dry them with a cloth and wait for the effects of hotness and coldness in your hands to subside. Next, place one hand at a time into basin B (your body temperature) they would feel neither hot nor cold.
Your left hand feels warm because its gain thermal energy from the lukewarm water in basin C. Your right hand feels cold because it loses thermal energy to the cold water in basin A. The water in basin B is at the same temperature as your hands – your hands feel neither hot nor cold.
Below is a video about the Misconception about Temperature:
A premium gasoline available at most stations has an octane rating of 92. What does that tell you about:
- The knocking characteristics of this gasoline?
- Whether the fuel contains oxygenates?
Answer:
- Gasoline with an octane rating of 92 means a knocking characteristic of 92% isooctane and 8% n-heptane. A premium gasoline which has a higher octane rating has lesser tendency to cause “knocking” in engines.
- The octane rating does not provide any information about whether the fuel contains oxygenates. This information should be available in other labels around the pump.
That’s all folks

