What is HAZE? HAZE is made up of adequate smoke, dust, moisture, and vapour suspended in relatively dry air. When weather conditions prevent the dispersal of smoke and other air pollutants, they concentrate and form a usually low-hanging shroud that impairs visibility and may become a respiratory health threat. Industrial pollution can result in a dense haze, which is known as smog. HAZE pollution can be said to be “transboundary” if its density and extent is so immense in a source that it remains at measurable levels after crossing into another country’s airspace.
What is AIR POLLUTANT? An air pollutant is a substance in the air that can have detrimental effects on humans and the ecosystem. The substance can be solid particles, liquid droplets, or gases. A pollutant can be of natural origin or man-made. Pollutants are classified as primary or secondary. Primary pollutants are harmful substances that are emitted directly into the atmosphere. Secondary pollutants are harmful substances formed in the atmosphere when a primary air pollutant reacts with substances normally found in the atmosphere or with other air pollutants. Some pollutants may be both primary and secondary: they are both emitted directly and formed from other primary pollutants.
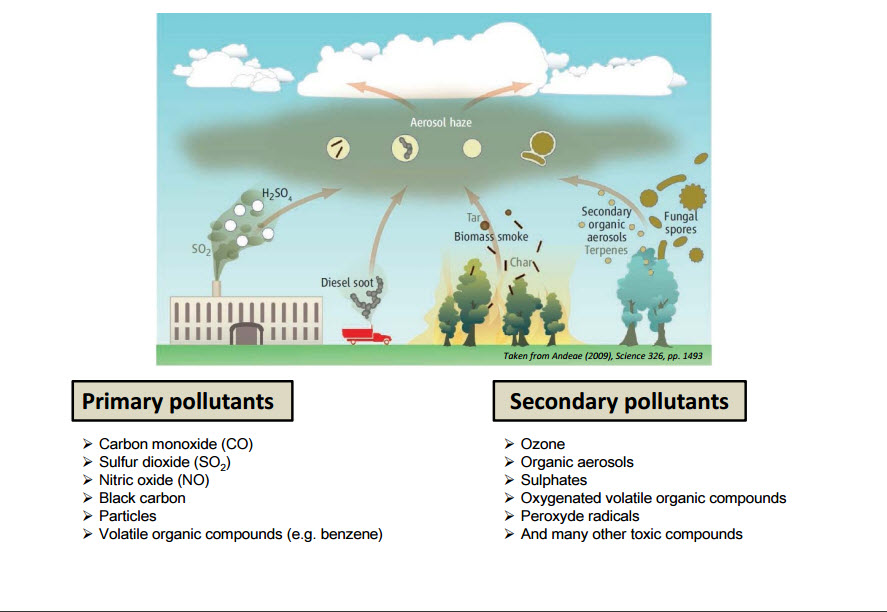
Major PRIMARY POLLUTANTS produced by human activity include:
- Carbon monoxide (CO) – CO is a colourless, odourless, toxic yet non-irritating gas. It is a product of incomplete combustion of fuel such as natural gas, coal or wood. Vehicular exhaust is a major source of carbon monoxide.
- Sulfur oxides (SOx) – particularly sulfur dioxide, a chemical compound with the formula SO2. SO2 is produced by volcanoes and in various industrial processes. Coal and petroleum often contain sulfur compounds, and their combustion generates sulfur dioxide. Further oxidation of SO2, usually in the presence of a catalyst such as NO2, forms H2SO4, and thus acid rain.This is one of the causes for concern over the environmental impact of the use of these fuels as power sources.
- Nitrogen oxides (NOx) – Nitrogen oxides, particularly nitrogen dioxide, are expelled from high-temperature combustion and are also produced during thunderstorms by electric discharge. They can be seen as a brown haze dome above or a plume downwind of cities. Nitrogen dioxide is a chemical compound with the formula NO2. It is one of several nitrogen oxides. One of the most prominent air pollutants, this reddish-brown toxic gas has a characteristic sharp, biting odour.
- Carbon dioxide (CO2) – This is by far the most emitted form of human-caused air pollution. Although CO2 is currently only about 405 parts per million in earth’s atmosphere, billions of metric tonnes of CO2 are emitted annually by burning of fossil fuels.The CO2 increase in earth’s atmosphere has been accelerating.
- Particulates, alternatively referred to as particulate matter (PM), atmospheric particulate matter, or fine particles, are tiny particles of solid or liquid suspended in a gas. In contrast, aerosol refers to combined particles and gas. Some particulates occur naturally, originating from volcanoes, dust storms, forest and grassland fires, living vegetation, and sea spray. Human activities, such as the burning of fossil fuels in vehicles, power plants and various industrial processes also generate significant amounts of aerosols. Averaged worldwide, anthropogenic aerosols—those made by human activities—currently account for approximately 10 percent of our atmosphere. Increased levels of fine particles in the air are linked to health hazards such as heart disease, altered lung function and lung cancer.
- Volatile organic compounds (VOC) – VOCs are a well-known outdoor air pollutant. They are categorised as either methane (CH4) or non-methane (NMVOCs). Methane is an extremely efficient greenhouse gas which contributes to enhanced global warming. Other hydrocarbon VOCs are also significant greenhouse gases because of their role in creating ozone and prolonging the life of methane in the atmosphere. This effect varies depending on local air quality. The aromatic NMVOCs benzene, toluene and xylene are suspected carcinogens and may lead to leukaemia with prolonged exposure. 1,3-butadiene is another dangerous compound often associated with industrial use.
SECONDARY POLLUTANTS include:
- Ground-level ozone (O3) formed from NOx and VOCs. Ozone (O3) is an essential constituent of the troposphere. It is also an important constituent of certain regions of the stratosphere commonly known as the Ozone layer. Photochemical and chemical reactions involving it drive many of the chemical processes that occur in the atmosphere by day and by night. At abnormally high concentrations brought about by human activities (largely the combustion of fossil fuel), it is a pollutant and a constituent of smog.
- Particulates created from gaseous primary pollutants and compounds in photochemical smog. Smog is a type of air pollution. Classic smog results from large amounts of coal burning in an area caused by a mixture of smoke and sulfur dioxide. Modern smog does not usually come from coal but from vehicular and industrial emissions that are acted on in the atmosphere by ultraviolet light from the sun to form secondary pollutants that also combine with the primary emissions to form photochemical smog.
- Peroxyacetyl nitrate (C2H3NO5) – similarly formed from NOx and VOCs.
References:
- Haze. Wikipedia. 2017 Mar 22 [accessed 2017 Apr 8]. https://en.wikipedia.org/wiki/Haze
- Vaswani K. Singapore haze hits record high from Indonesia fires. BBC News. 2013 Jun 21 [accessed 2017 Apr 8]. http://www.bbc.com/news/world-asia-22998592
- About Haze. About Haze. [accessed 2017 Apr 8]. http://www.haze.gov.sg/about-haze







