Introducing pH

What is pH?
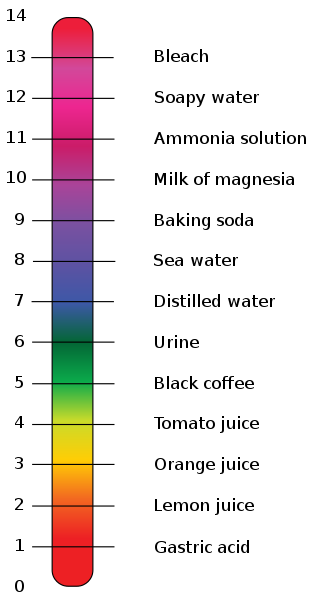
“pH” is an abbreviation for “Power of Hydrogen“. It is a logarithmic scale measuring the concentration of hydrogen ions in a water-based solution. The pH scale runs from 1-14. Substances with a pH of 1 to 6 are acidic, while substances with a pH of 8 to 14 are basic (alkaline). Substances with a pH of 7 are neutral. Lemon juice is acidic (pH 2), bleach is alkaline (pH 13), and pure water is neutral (pH 7).
Why is this topic important?
Maintaining the correct pH is essential for living things, as many living things can only exist around a narrow range of pH values. For example, the normal pH of human blood is 7.4. If the pH falls below 6.8 or rises above 7.8, death occurs. Even inanimate objects rely on maintenance of correct pH to function properly. For example, soil should have a pH of 7 to ensure optimal growth of plants. Wrong harvesting methods or overuse of fertilizers can change the soil pH and the soil then needs to be treated with chemicals to restore the pH to the optimal level.
Knowledge of pH is thus essential for maintaining the proper acidity or basicity in various products. It is thus very applicable to our daily lives and has a large impact on society as everyone relies not just on correct pH to stay alive and healthy, but also on products whose pH is at the correct level.
References:
- Acid, Base & Salt. (n.d.). Retrieved March 08, 2017, from http://10upon10.com/10thscience-acid-base-salt-11.html
- Helmenstine, A. M. (2017, February 13). What Does pH Stand For? Retrieved March 8, 2017, from https://www.thoughtco.com/what-does-ph-stand-for-608888
- Stevens, E. (2010, March 29). PH Scale.svg [A pH scale with annotated examples of chemicals at each integer pH value.]. Retrieved April 4, 2017, from https://upload.wikimedia.org/wikipedia/commons/thumb/a/a1/PH_Scale.svg/317px-PH_Scale.svg.png