Introduction
- Total energy consumption 2019-2014. (2016, May 18). Retrieved March 29, 2017, from https://www.ema.gov.sg/cmsmedia/Publications_and_Statistics/Statistics/13RSU.pdf
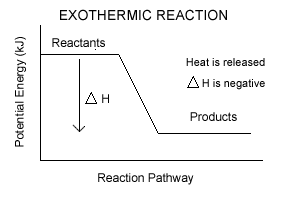
Chemical Concept
- U.S. Energy Information Administration – EIA – Independent Statistics and Analysis. (n.d.). Retrieved March 29, 2017, from https://www.eia.gov/tools/faqs/faq.cfm?id=73&t=11
- Hydrocarbon combustion. (n.d.). Retrieved March 29, 2017, from http://energyeducation.ca/encyclopedia/Hydrocarbon_combustion#cite_note-3
- Reporter, S. (2016, February 11). World Energy Day 2014: How Much Oil is Left and How Long Will it Last? Retrieved March 29, 2017, from http://www.ibtimes.co.uk/world-energy-day-2014-how-much-oil-left-how-long-will-it-last-1471200
- Fossil Fuels. (n.d.). Retrieved March 29, 2017, from http://www.chemistryexplained.com/Fe-Ge/Fossil-Fuels.html#ixzz4bNfGlCOA
Implications
- Society, N. G. (2013, January 14). Petroleum. Retrieved March 29, 2017, from http://www.nationalgeographic.org/encyclopedia/petroleum/
- Nwf. “How You Can Help Wildlife Impacted by the BP Oil Spill – National Wildlife Federation.” N.p., n.d. Web. 29 Mar. 2017.
- Adebayo, Abdulrauf Rasheed, and Bassam Tawabini. “Hydrocarbon Exploration and Production- a Balance between Benefits to the Society and Impact on the Environment.” OMICS International. OMICS International, 27 Apr. 2012. Web. 29 Mar. 2017.