Introduction
Our group will be focusing on Polymers as our chosen topic. A polymer is a large molecule or a macromolecule formed by joining many repeated subunits. They may be naturally found in plants and animals known as the natural polymers or may be man-made called the synthetic polymers. Different polymers have a number of unique physical and chemical properties due to which they find usage in everyday life.
Polymers make up of many materials in living organisms, including proteins, cellulose and nucleic acids. Moreover, they constitute the basis of such minerals as diamond, quartz and man-made materials as concrete, glass, paper etc.
Polymers make up so many things that we can find in our surroundings, one of them is our DNA where it is a polymer. Using general terms, polymers are often named as plastic. With the use of the material plastic, it is able to create many different things and types such as a shopping bag. The polymer for a shopping bag is significantly different from the polymer used to make a PVC which is different from that to make polystyrene.
Polymers are used daily in our lives, such as polystyrene which is one of the most common plastic, actively used in the packaging industry. Bottles, toys, containers etc are some of the daily-used products made up of polystyrene, where it is used as an insulator. PVC is commonly used in clothing and furniture and has been popular for the construction of doors and windows as well.
Chemical Concept
Introduction
- A polymer consists of long chain molecules, each molecule made up of repeated units connected together.
- Polymers are synthesized by many small molecules joining together into very large molecules called macromolecules. The small units called monomers, are unsaturated organic molecules.
- Polymers can be synthesised in 2 kinds of reactions: addition polymerisation and condensation polymerisation.
- In addition polymerisation, the reaction involves the formation of free radicals to initiate the polymerisation.
- Condensation polymerisation usually involves the interaction between the side functional groups, leading to the formation of by-products.
- When two or more different monomers unite together to polymerize, their result is called a copolymer and its process is called copolymerization. Fig 1 shows the possible type of co-polymers.
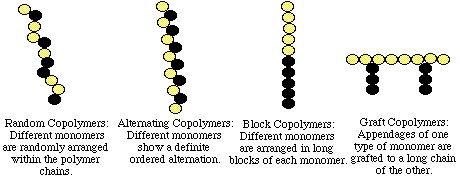
Fig 1: Types of co-polymers
Molecular Arrangement of Polymers chains

Fig 2: Arrangement of polymer chains
- Controlling the cooling rate of polymer melt can result in different arrangement of the polymer chains
- When quenching is used( i,e rapid cooling), an amorphous arrangement of molecules is obtained since the polymer chains do not have enough time to rearrange the chains.
- Due to the lack of arrangement, light can pass through amorphous material without being refracted, hence Amorphous polymers are generally transparent.
- On the other hand, with slow cooling, a high degree of crystallinity can be achieved. Polymers with high degree of crystallinity appears translucent and opaque since light is refracted by the regular array of crystal.
Characteristics of Polymers
Every polymer has very distinct characteristics, but most polymers have the following general attributes.
- Polymers can be very resistant to chemicals.
- Polymers can be both thermal and electrical insulators.
- Polymers are very light in weight with significant degrees of strength.
- Polymers can be processed in various ways.
- Polymers are materials with a seemingly limitless range of characteristics and colors.
- Polymers are usually made of petroleum, but not always.
References: https://plastics.americanchemistry.com/plastics/The-Basics/
Implications on Society
Introduction
Polymers are found in every corner of our modern living world and brings about immense convenience and advantages to our lives. However, there are some negative impacts of their presence. Plastic, a commonly used polymer, occupy huge volumes in landfills and pose a serious problems for all the countries in the world. Large amounts of them have been found in the oceans as well. The plastic fragments had most likely leached into the drains during rain, transported into the rivers and ultimately into the seas and oceans.
Polymers are dumped in various landfills in many countries. In the landfills, the polymers take a very long time to biodegrade. That is because of the lack of oxygen and moisture in the layers below the surface. Biodegradable polymers can be defined as polymers that can be degraded directly by the action of the enzymes under normal conditions like in room temperature or under aerobic conditions. The main degrading organisms are bacteria, fungi, archaea and even insects.
Since the polymers will be degraded one day and its components returned to the environment, precautions must be taken to not use toxic materials whilst production. We should always consider the environmental impacts from the production of the polymer to the degradation process.
Explanation
Polyvinyl chloride (PVC), one of the most commonly used plastics in the world, exceeds 35 million tonnes of usage in the world per year. It has vast applications, ranging from cables and flooring to pipes and fittings. PVC degrades easily under the action of light and heat and releases toxic substances. In the industries, plasticizers which are based on phthalate are used to make PVC more flexible. This substance posed chronic toxicity to animals and has teratogenic effects. It also poses body growth problems and causes reproduction complications in humans. The small fragments of PVC can evaporate and rise to the stratosphere to destroy the ozone layer. During disposal, if PVC were to be incinerated, toxic products such as dioxins are produced and this poses another threat to human health.
Another common polymer is polycarbonate (PC). They are a naturally transparent amorphous thermoplastic. The advantage of using PC is that it allows for the internal transmission of light to be nearly in the same capacity as glass. PC polymers are common used to product plastic lenses in eyewear or protective gears due to its transparency. During the production of PC plastic, a chemical known as bisphenol A is used as one of its monomers. It has been shown that this chemical is toxic and poses carcinogenic effects and neurotoxicity in animals. During decomposition of the resin, these toxic monomers can be released into the environment.
Biopolymers generally have little impact on the environment. They are the polymers produced by living organisms and have been around for millions of years. The only implication it might bring about is during degradation under anaerobic conditions. This process generates biomass, methane, carbon dioxide, water and some other small molecules. The issue presented is with the methane as it contributes to the greenhouse gases. Biopolymers will act as a contaminant upon mixing with other polymers during recycling. Biopolymers are not recyclable and usually degrade during recycling processes.
References
