Qn 1) From personal experience, state whether these processes are endothermic or exothermic. Give a reason for each.
- A charcoal briquette burns.
Exothermic. As a charcoal briquette burns, it releases heat. - Water evaporates from your skin.
Endothermic. Water absorbs the heat necessary for evaporation from your skin, and your skin feels cooler.
- Ice melts.
Endothermic. Ice absorbs the necessary heat from the environment to melt.
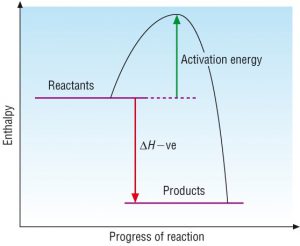
Qn 2) Chemical explosions are very exothermic reactions. Describe the relative bond strengths in the reactants and products that would make for a good explosion.
A good explosion is defined as one that produces a lot of energy. Explosions are exothermic reactions as they generate energy. For exothermic reactions, the amount of energy released from forming bonds is more than the energy absorbed from breaking the bonds. The relative bond strength is directly proportional to the bond energy. Therefore, the magnitude of the bond strength for the products must be larger than that of the reactants, to lead to an enthalpy change that is as negative as possible which would then lead to a good exothermic reaction and hence a good explosion.
Qn 3) How might you explain the difference between temperature and heat to a friend? Use some practical, everyday examples?
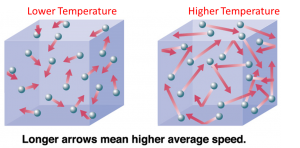
Temperature is a measure of the average kinetic energy of the particles in an object, it determines how ‘hot’ or ‘cold’ something is. On the other hand, heat is energy that flows from a hotter to a colder region.
Example: There are two cups of water one at 25°C while the other is at 70°C. The one at 70°C would have a higher temperature due to the average kinetic energy in the water being higher than that of the one at 25°C. When you mix both liquids together, heat will be transferred from the water at 70°C to the water at 25°C due to the temperature difference.
Qn 4) A premium gasoline available at most stations has an octane rating of 98. What does that tell you about:
- the knocking characteristics of this gasoline?
A premium gasoline with an octane rating of 98 means that it has a knocking characteristics of 98% isooctane and 2% heptane.
- whether the fuel contains oxygenates?
If the fuel contain oxygenates, it should give an octane rating of >100, since the oxygenates such as ethanol and MTBE (methyl tertiary-butyl ether) will raise the octane rating to make it better than the “standard”, which is isooctane with an octane rating of 100.