The 1962 thalidomide disaster is one of the darkest events in the history of pharmaceutical research. The drug was marketed as a ‘wonder drug’ to treat a range of conditions that was supposedly safe, even for pregnant women. However, the drug’s teratogenic S-enantiomer caused thousands of children in the 1960’s to be born with malformed limbs. Ever since the tragedy surrounding thalidomide, the world has been made more aware of the significance of drug isomerism, as isomers of the same compound can differ in their pharmacokinetic and pharmacodynamic properties. This page explains how drug isomerism in thalidomide has directly impacted our society.
I. Introduction
Molecular chirality was discovered in 1848 by Louis Pasteur, a French chemist and biologist, when he successfully separated the two enantiomers of sodium ammonium tartarate for the first time. However, it was only after the 1962 thalidomide disaster that drug researchers began to realize the importance of molecular chirality on drug activity. Although the enantiomers of chiral drugs have the same chemical connectivity of atoms, they tend to differ greatly in their pharmacokinetic and pharmacodynamic properties.
One of the reasons why chiral drugs have different biological activities has to do with the recognition of the enantiomers by specific drug receptors found in enzymes and proteins that can be explained by a three-point interaction of the drug with the receptor site that was proposed by Easson and Stedman during the 20th century.
Essentially, the Easson-Stedman hypothesis states that only a particular enantiomer that has a complementary shape to the receptor site can fit into a receptor site to produce an active biological effect. The other enantiomer will not fit in the same way with its receptor but may fit into a receptor site elsewhere in the body, causing an unwanted or toxic effect.
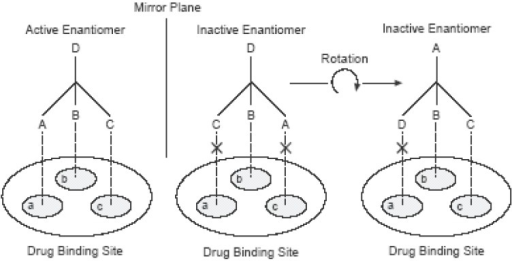
Easson-Stedman hypothetical interaction between the two enantiomers of a chiral drug with a receptor at the drug binding sites.
In the case of thalidomide, it can undergo a wide spectrum of mechanisms within the human body to produce a number of chiral and achiral metabolites, most of which are not fully understood. In addition, thalidomide’s chiral carbon is unstable; it can interswitch between the two enantiomers rapidly in bodily fluids and in water to form a racemic mixture, making is difficult to determine exactly the biological effect of each enantiomer. However, most studies have concluded that its R-enantiomer is an effective sedative, whereas its S-enantiomer is a teratogen (a substance affecting the development of the embryo or foetus, causing structural or functional disability).

The phthalimide ring is believed to be responsible for teratogenic effects whereas the gluarimide ring resembles a structure similar to hypnotic drugs and is believed to be responsible for sedative properties.
Thalidomide was notoriously known to be responsible for the following birth defects: amelia (absence of limbs), phocomelia (absence of most of the arm with hands extending flipper-like from the shoulders), dysmelia (malformation, missing or extra limbs), bone hypoplasticity (incomplete development/below normal number of cells), and other congenital defects of the ear, heart, and internal organs.

Thalidomide’s teratogenic S-enantiomer caused thousands of children in the 1960’s to be born with malformed limbs.
Other adverse side effects of thalidomide include: drowsiness, constipation, rash, nausea, decreased thyroid activity, increased appetite, muscle weakness, and menstrual abnormalities.
II. Socio-Economic Costs
Today, the number of thalidomide survivors alive around the world is estimated to be around 5,000. Even though nearly 60 years have passed since the tragedy, the socio-economic costs of thalidomide can still be felt today.
For the survivors, decades of compensating for their disabilities has meant greater wear and tear on their body structure, leading to further physical deterioration and the premature onset of other ailments like arthritis and chronic pain. The special adaptations that these survivors require in their homes and cars also tend to be very expensive due to the rarity of their conditions, further compounding their problems.

In order for her to maintain independence and mobility, a large car is needed to provide space for the motorised wheelchair and an electric hoist is deployed to get the wheelchair into the car.
Most of the survivors who were able to join the workforce have already been forced into early retirement, while others who used to rely on their parents for constant care, can no longer do so. This places an increasing strain on society as more thalidomiders are becoming more dependent on other family members, on social benefits, on health insurance payouts, or on charity.
Among the survivors today, nearly half of them fall under the compensation deal with Germany. However, the yearly payouts (£11,840 maximum) they receive are insufficient to cover the needs of those with multiple limb deficiencies, have no independent income and require constant care.
The needs and problems of this unique population are many and overwhelming, but the situation is becoming more hopeful in some countries, with governments providing more security by guaranteeing long-term financial support for growing health needs of the survivors.
III. Safer and More Effective Drug Alternatives
The discovery of stereoisomerism in drugs has opened a new challenge and field in clinical pharmacology. Before the 1990s, about half the drugs in the market were of racemic mixture. A racemic mixture is a drug that contains 2 or more enatiomers. In some cases like the Thalidomide incident, the two isomers in the racemic mixture of a drug can be of agonist and antagonist type, resulting in the drug to be less effective or undesirable for application.
Hence, more focus is put into creating safer and effective drugs, with many existing drugs undergoing the chiral switch. Currently, a large number of clinical trials are ongoing to see the effect of single enatiomers drugs over its racemic mixture counterpart. Single enatiomers have less complex and more selective pharmacodynamic profile, meaning it has a less adverse drug reaction, improved therapeutic profile, and less chances of drug interactions than racemic mixtures. The use of single enatiomers in drugs put forth greater benefits for patients, where the antagonist isomers are eliminated and detrimental drug reaction are avoided, and he/she will be exposed a lesser amount of drugs, overall increasing his/her metabolic health.
An example of a drug conversion is Centrizine(racemic mixture) to levocetrizine(single enatiomers). Even though both confers the same antihistamine functions, the normal Centrizine pill contains both dextro rotatory cetrizine and levo rotatory cetrizine, in which the dextro isomer has no effect in treatment of the ailment. Thus, levocentrizine isolates only the active levo isomers, essential in treating the ailment. It also removes side effects caused by the dextro rotatory centrizine. Other single enatiomers drugs available in the market are Escitalopram, Naproxen, Levosalbutamol, etc.
IV. Improvements in Testing and Approval Processes
Prior to the drug crisis, clinical trials in most countries were not subjected to the supervision by a regulating authority. The “clinical trials” of thalidomide in the United States involved the dispensing of thalidomide to more than 20,000 unknowing patients (of which 207 were pregnant) in the country and did not include any followup.
Animal tests were not done on pregnant animals; at that time, the placenta was believed to be an effective barrier to drug transfer. Hence there was insufficient information as to whether thalidomide could cross the placenta and affect fetal development.
The wise refusal to approve thalidomide for sale in the United States by the Food and Drug Administration due to the incomplete and insufficient data on the safety and effectiveness of thalidomide helped to reduce the scale of the catastrophe.

Frances Kelsey, the heroin who prevented the sale of thalidomide in the United States despite pressure from pharmaceutical companies and her supervisors, being presented with a medal of service by President John F. Kennedy for her vigilance.
As a result of the worldwide thalidomide disaster, countries around the world, including the United States, made improvements to their drug testing and approval processes. In the United States, the Kefauver-Harris Drug Amendments Act, which was passed almost immediately after the thalidomide disaster, required drug manufacturers to prove that their drugs are both safe and effective before they can be sold.
Today, the approval of drugs can take up to twelve years, involving animal testing and tightly regulated human clinical trials.
V. Usage of Thalidomide Today
Although thalidomide was withdrawn from the market in 1962 due to its harmful side effects, there is renewed interest in its controlled use today.
Research have shown that thalidomide exhibits immunomodulatory, anti-angiogenic, and anti-inflammatory properties that can be very useful in the treatment of certain illnesses.
It has the ability to affect the functioning of the immune system, inhibit the growth of new blood vessels, reduce inflammation or swelling as well as the ability to inhibit tumour growth.
Today, thalidomide is used in the treatment of leprosy and myeloma. However, because of its known teratogenic effects, the distribution of thalidomide is tightly regulated and patients are made aware of its potential risks before taking the drug.
VI. Conclusion
The advancement in our understanding of drug isomerism has helped us in the development of safer and more effective drug alternatives to many existing drugs as well as improvements in the testing and approval processes of new drugs.
Hopefully, with our increasing knowledge in drug chemistry, instances of such medical disasters can be reduced.
References
Chhabra, N., Aseri, M. L., & Padmanabhan, D. (2013). A review of drug isomerism and its significance. International Journal of Applied and Basic Medical Research, 3(1), 16–18. http://doi.org/10.4103/2229-516X.112233
Vargesson, N. (2015). Thalidomide‐induced teratogenesis: History and mechanisms. Birth Defects Research, 105(2), 140–156. http://doi.org/10.1002/bdrc.21096
Nguyen, L. A., He, H., & Pham-Huy, C. (2006). Chiral Drugs: An Overview. International Journal of Biomedical Science : IJBS, 2(2), 85–100. http://doi.org/10.4103/2229-516X.112233
Blake K., Raissy H. (2013).Chiral Switch Drugs for Asthma and Allergies: True Benefit or Marketing Hype. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3777548/
National Center for Biotechnology Information. PubChem Compound Database; CID=5426, https://pubchem.ncbi.nlm.nih.gov/compound/5426 (accessed Mar. 28, 2017).
Optical Isomerism In Thalidomide. (n.d.). Retrieved from http://www.chm.bris.ac.uk/motm/thalidomide/optical2iso.html
Fintel, B., Samaras, A. T., & Carias, E. (2009, July 28). THE THALIDOMIDE TRAGEDY: LESSONS FOR DRUG SAFETY AND REGULATION. Retrieved from https://helix.northwestern.edu/article/thalidomide-tragedy-lessons-drug-safety-and-regulation
Chiral drugs. (n.d.). Retrieved from https://www.khanacademy.org/test-prep/mcat/chemical-processes/stereochemistry/a/chiral-drugs
Anderson, J. D. (2013, May 20). Thalidomide. Retrieved March 28, 2017, from http://www.toxipedia.org/display/toxipedia/Thalidomide
Lauranc, J. (2012, December 21). GOVERNMENT’S £80M FOR VICTIMS OF THALIDOMIDE – BUT STILL NO APOLOGY. Retrieved from http://www.independent.co.uk/life-style/health-and-families/health-news/government-s-80m-for-victims-of-thalidomide-but-still-no-apology-8427855.html
Dove, F. (2011, November 3). What’s happened to Thalidomide babies? Retrieved from http://www.bbc.com/news/magazine-15536544
Hamburg, M. (2012, February 7). 50 Years after Thalidomide: Why Regulation Matters. Retrieved from https://blogs.fda.gov/fdavoice/index.php/2012/02/50-years-after-thalidomide-why-regulation-matters/
Davies, M., & Kerimani, F. (2016, November 4). Overviews on FDA History FDA and Clinical Drug Trials: A Short History. Retrieved March 28, 2017, from https://www.fda.gov/%20AboutFDA/WhatWeDo/History/Overviews/ucm304485.htm

