Category: Research
Biofuels
Carbon Fixation
The process of taking an inorganic compound e.g. Carbon Dioxide to organic compounds. If it happens in a living organism its called biological carbon fixaton.
Biofuel
A hydrocarbon by or from a living organism that humans can use to power something e.g. cars or any hydrocarbon produced by or from a living organism in a short period of time.
Biomass
Organic matter, dead material that was once living e.g. kernel of corn sugar cane etc. It is used to make biofuels and this makes biofuel more renewable as biomass can be reproduced in a short time.
Examples of biofuel vs its fossil fuel counterparts
| Biofuel | Fossil Fuel | Differences |
| Ethanol | Ethane/ Gasoline | Ethanol burns cleaner than gasoline but it only has half the energy per unit mass of gasoline. Thus it produces less carbon monoxide but more ozone which contributes substantially to smog |
| Biodiesel | Diesel | Burns cleaner than diesel, produce less particulate and fewer sulfur compounds. However, it is corrosive to the engine thus modifications have to be done for the engine to take biodiesel. Has only slightly less energy per unit mass than diesel |
| Methanol | Methane | Easier to transport as compared to methane as it’s in the liquid form. Only has one third to half as much energy per unit mass as methane. |
| Biobutanol | Butane/ Gasoline | Can run in any car that uses gasoline, contains almost the same energy per unit mass as gasoline |

Alcohols contain very little or no sulfur thus combustion of alcohol produces very little sulfur dioxide and sulfuric acid. However, alcohols are derived from plant and animal matter thus containing a lot of nitrogen. This is bad as it will produce a lot of nitric oxides and other nitrogenous compounds which contributes to acid rain in the long run
However, burning alcohols and biodiesel produces less carbon monoxide as compared to petrol diesel.
Production of biofuels from biomass
Biofuel: Ethanol
Biomass: sugar cane, corn, wheat, sugar beets, industrial sweet potatoes

Method of production: Biochemical Conversion
Enzymes and microorganisms Catalyse the conversion of biomass or biomass-derived compounds into desirable products. Enzymes such as Cellulase break down the carbohydrate fractions of biomass to five- and six-carbon sugars via hydrolysis, Yeast and bacteria then ferment the sugars into products
https://energy.gov/eere/energybasics/articles/biofuel-conversion-basics
http://www.eesi.org/topics/bioenergy-biofuels-biomass/description
Ozone Layer
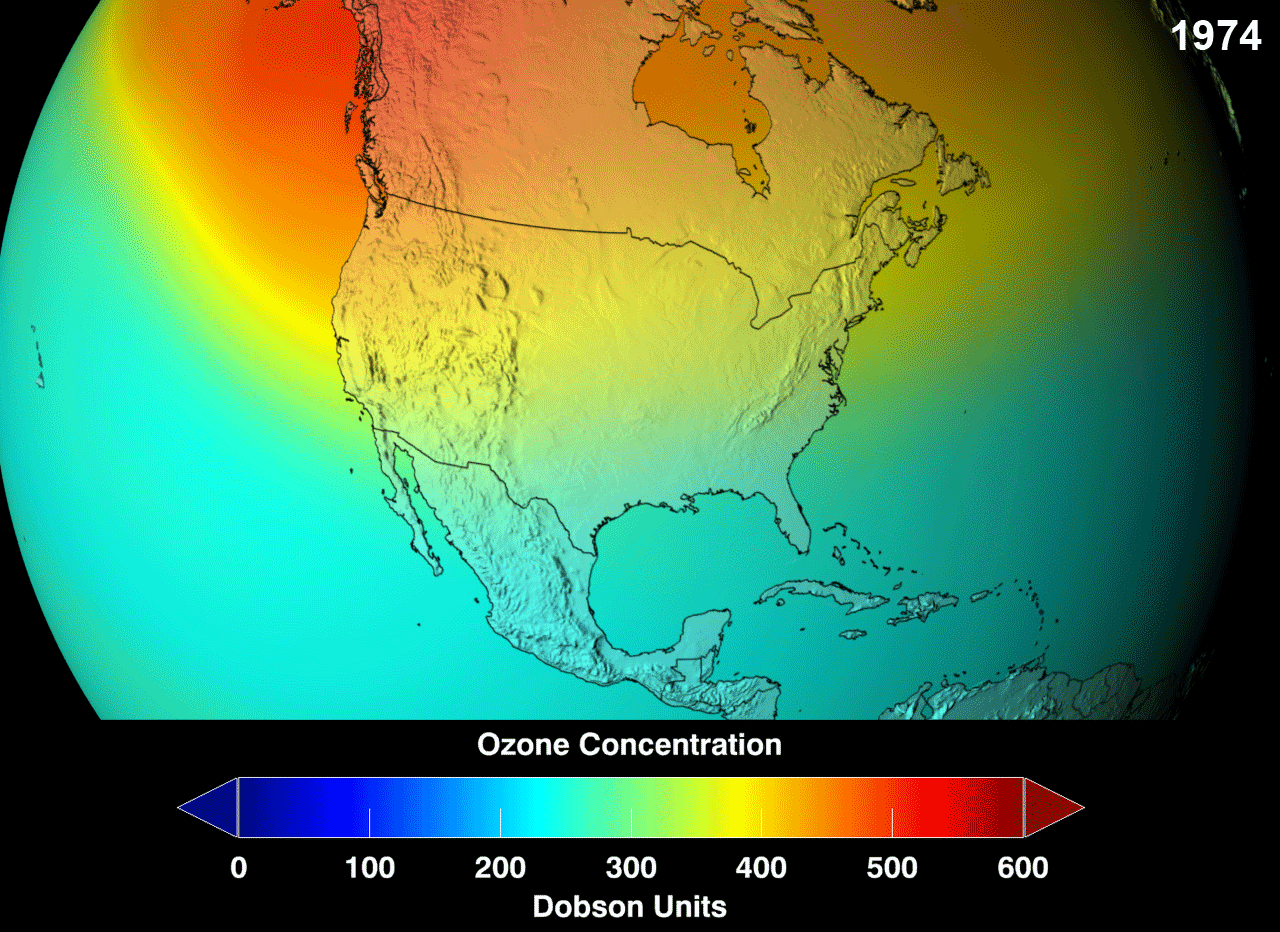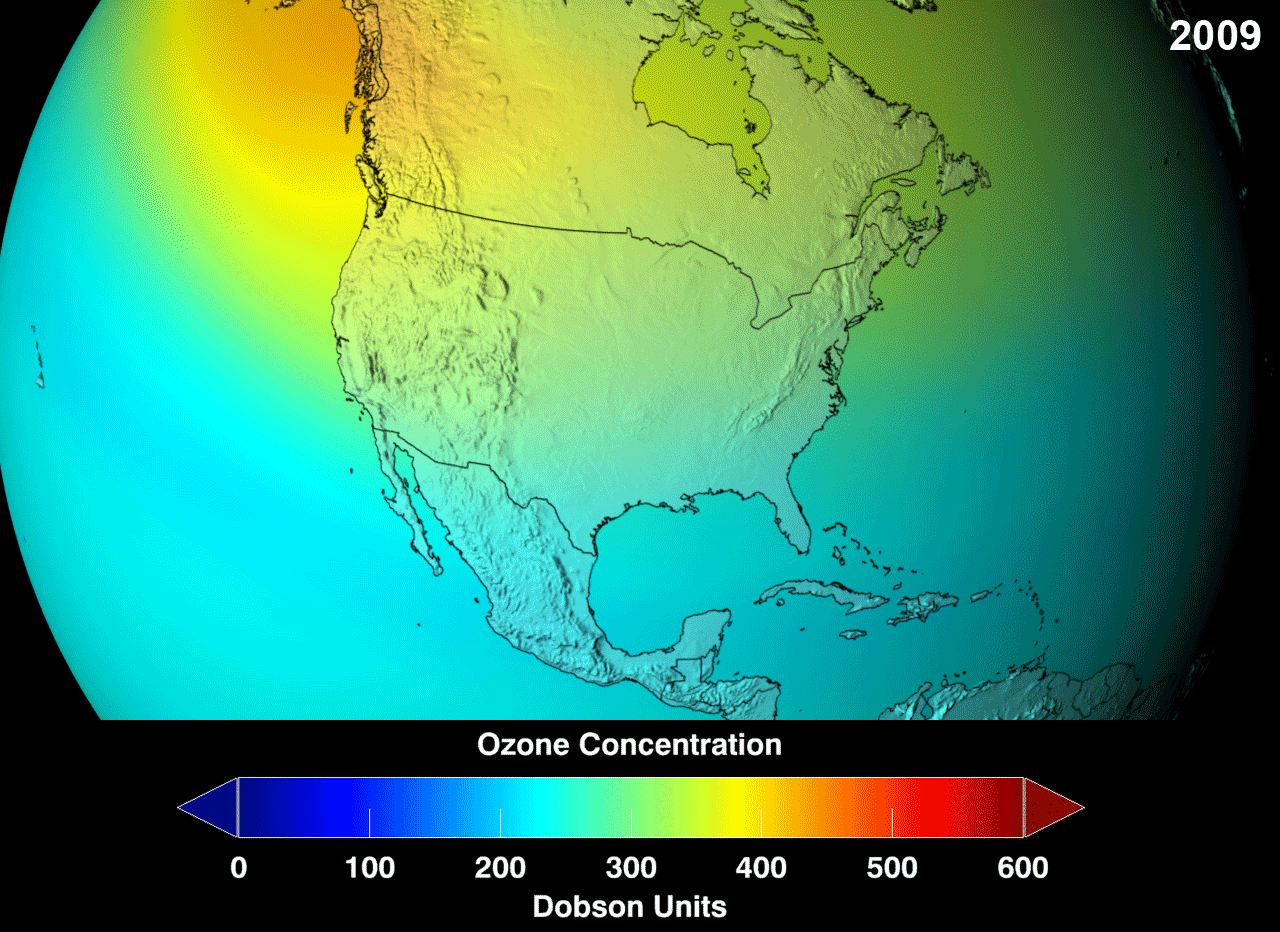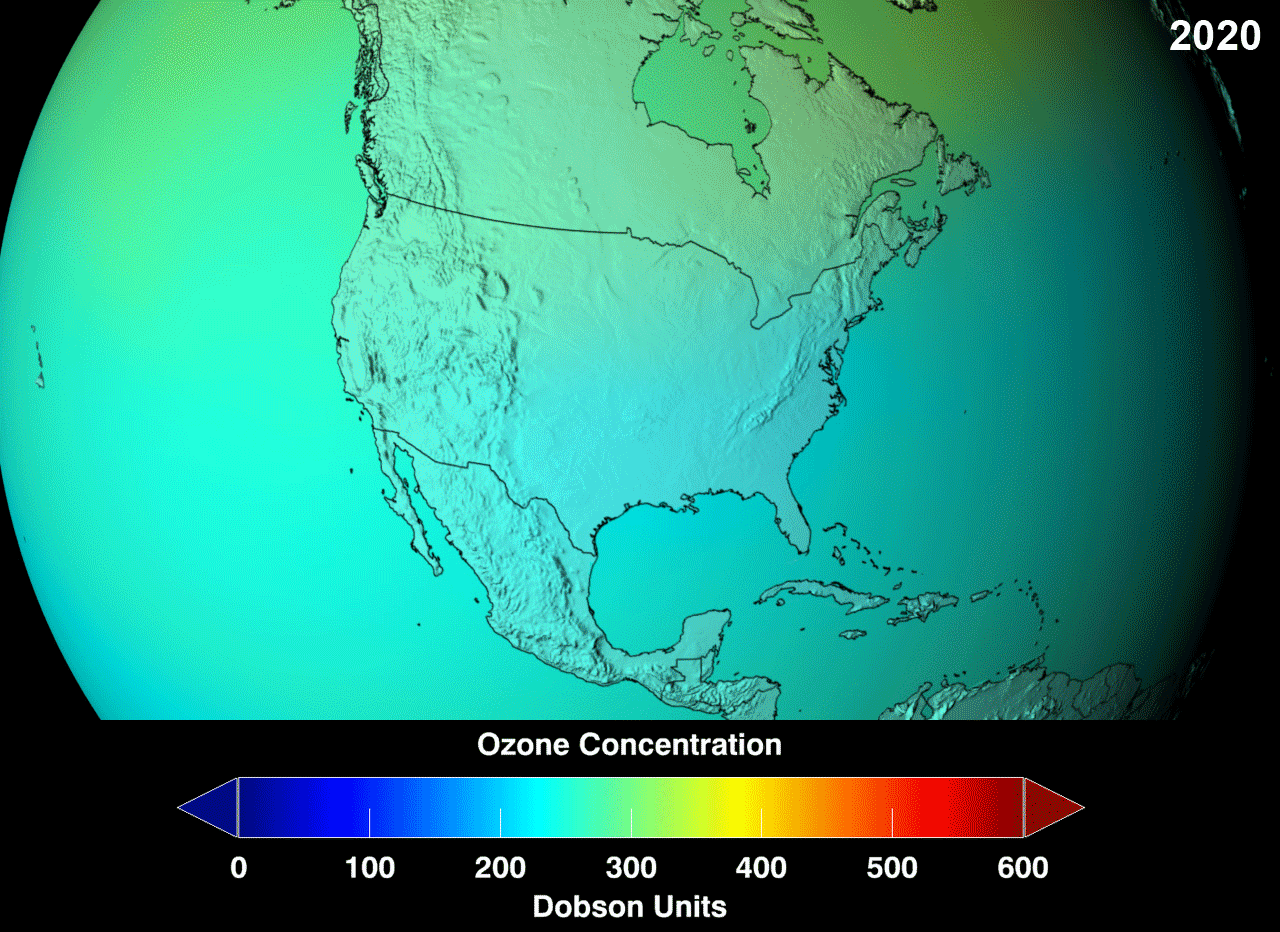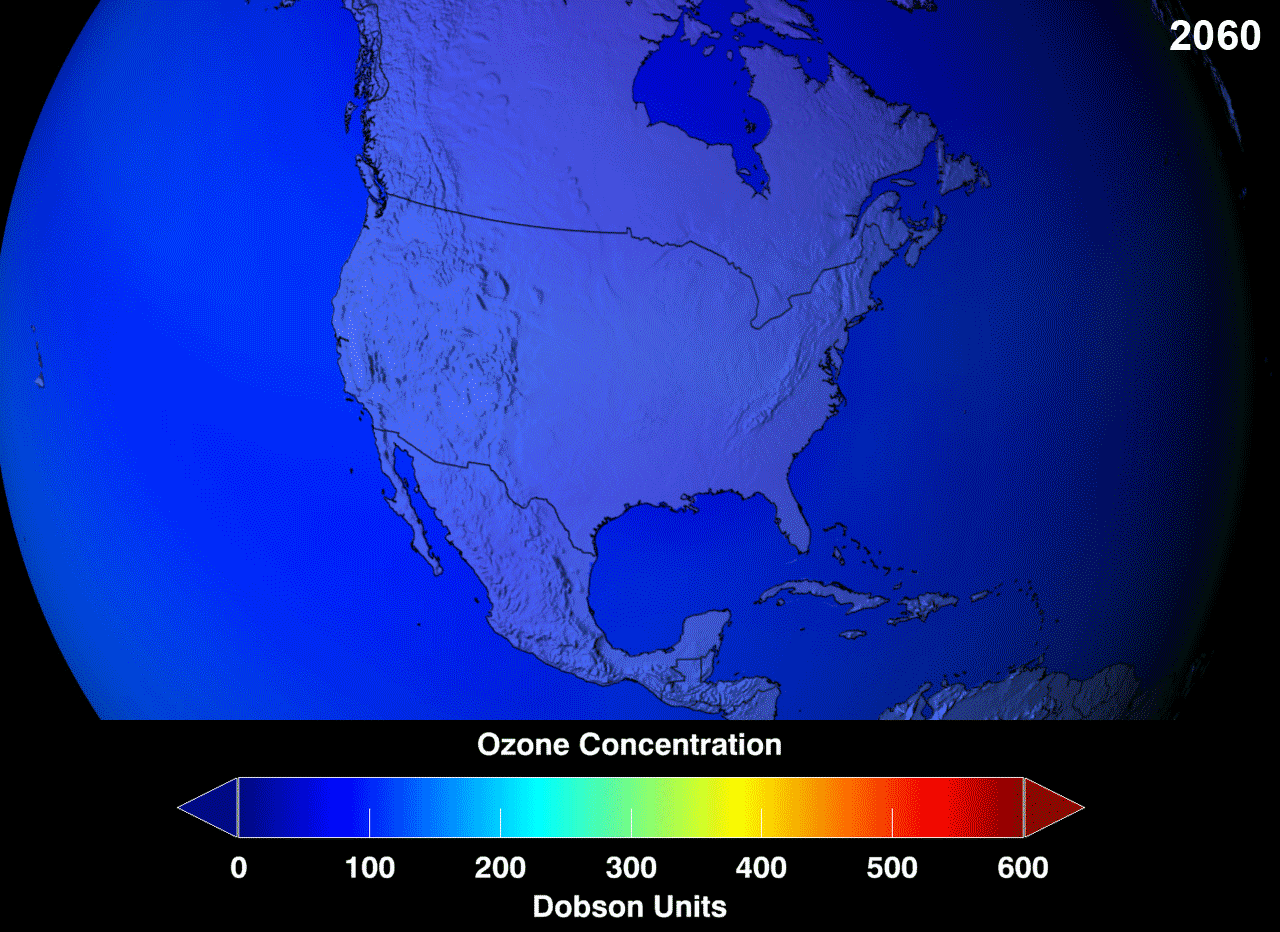
Definition
The ozone layer or ozone shield is a region of Earth’s stratosphere that absorbs most of the Sun’s ultraviolet (UV) radiation. It contains high concentrations of ozone (O3) in relation to other parts of the atmosphere, although still small in relation to other gases in the stratosphere. The ozone layer contains less than 10 parts per million of ozone, while the average ozone concentration in Earth’s atmosphere as a whole is about 0.3 parts per million. The ozone layer is mainly found in the lower portion of the stratosphere, from approximately 20 to 30 kilometres (12 to 19 mi) above Earth, although its thickness varies seasonally and geographically.

The ozone layer absorbs 97 to 99 percent of the Sun’s medium-frequency ultraviolet light (from about 200 nm to 315 nm wavelength), which otherwise would potentially damage exposed life forms near the surface.
Fun Fact: The United Nations General Assembly has designated September 16 as the International Day for the Preservation of the Ozone Layer.
When was the ozone layer discovered?
The ozone layer was discovered in 1913 by the French physicists Charles Fabry and Henri Buisson. Measurements of the sun showed that the radiation sent out from its surface and reaching the ground on Earth is usually consistent with the spectrum of a black body with a temperature in the range of 5,500–6,000 K (5,227 to 5,727 °C), except that there was no radiation below a wavelength of about 310 nm at the ultraviolet end of the spectrum. It was deduced that the missing radiation was being absorbed by something in the atmosphere. Eventually the spectrum of the missing radiation was matched to only one known chemical, ozone. Its properties were explored in detail by the British meteorologist G. M. B. Dobson, who developed a simple spectrophotometer (the Dobsonmeter) that could be used to measure stratospheric ozone from the ground. Between 1928 and 1958, Dobson established a worldwide network of ozone monitoring stations, which continue to operate to this day. The “Dobson unit”, a convenient measure of the amount of ozone overhead, is named in his honor.
Formation of ozone
Ozone present in the stratosphere forms a protective layer, known as ozone layer , ozonosphere or ozone umbrella. Its concentration in atmosphere is about 10 ppm. In the upper atmosphere, atmospheric gases absorbs the sun’s radiation and release molecules . In lower atmosphere , atmospheric oxygen get dissociated and subsequently combines with molecular oxygen of the upper stratosphere, thereby producing ozone.
O2—–(UV radiation)—-> O+O
O2 + O ———–> O3
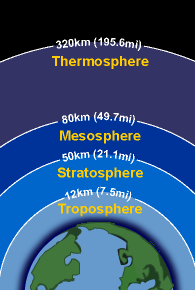
Different layers of the atmosphere
(Adapted from: http://www.conserve-energy-future.com/ozone-layer-and-causes-of-ozone-depletion.php)
To understand ozone layer, it would be helpful to know the different layers of the atmosphere. The earth’s atmosphere is composed of many layers, each playing a significant role. The first layer stretching approximately 10 kilometers upwards from the earth’s surface is known as the troposphere. A lot of human activities such as gas balloons, mountain climbing, and small aircraft flights take place within this region.
The stratosphere is the next layer above the troposphere stretching approximately 15 to 60 kilometers. The ozone layer sits in the lower region of the stratosphere from about 20-30 kilometers above the surface of the earth. The thickness of the ozone layer is about 3 to 5 mm, but it pretty much fluctuates depending on the season and geography.
Ozone layer is a deep layer in earth’s atmosphere that contain ozone which is a naturally occurring molecule containing three oxygen atoms. These ozone molecules form a gaseous layer in the Earth’s upper atmosphere called stratosphere. This lower region of stratosphere containing relatively higher concentration of ozone is called Ozonosphere. The Ozonosphere is found 15-35 km (9 to 22 miles) above the surface of the earth. The thickness of the ozone layer differs as per season and geography. The highest concentrations of ozone occur at altitudes from 26 to 28 km (16 to 17 miles) in the tropics and from 12 to 20 km (7 to 12 miles) towards the poles.
Importance of Ozone
(Adapted from: http://jontymagicman.expertscolumn.com/article/importance-ozone-layer-environment)
The presence of ozone layer in the stratosphere is vital as it absorbs the ultraviolet radiation that is harmful to life and prevent them from reaching the Earth’s atmosphere . If these radiations are allowed to reach the Earth’s atmosphere they will increase the temperature of lower atmosphere to such an extent , that it will be impossible for any life to survive on earth. In addition, these UV radiations can cause severe radiation damage in human and animals such as DNA mutation and skin cancer.
O3 + UV radiations——–> O2 + O
Depletion of Ozone
Mechanism of ozone depletion includes:
Natural process : Ozone in the upper atmosphere absorbs UV radiations of short wavelength and release atomic oxygen. This natural mechanism however do not upset the equilibrium of ozone because atmospheric oxygen absorbs UV radiation of wavelength of shorter than 240nm and photo dissociates into two oxygen atoms. This oxygen combines O2 molecules to form ozone.
Anthropogenic Process: CFCs used in refrigerants and aerosol sprays destroy ozone molecules. At the stratosphere, CFCs breaks to produce chlorine radicals. These chlorine radicals react with ozone molecules and destroy the ozone molecules in a series of reaction:
CFCl3 + UV Light ——- > CFCl2 + Cl
Cl + O3 ———– > ClO + O2
ClO + O ———– > Cl + O2
The free chlorine radical produced will react and destroy another ozone molecule again.
Impacts of CFCs on ozone layer

In 1985, a hole the size of the United States appeared in the ozone layer above the Antarctic. Without the ozone layer, more UV-B rays from the sun penetrate the atmosphere with various inconvenient results, such as a massive increase in skin cancers, reduced crop productivity, depletion of fish stocks, and climate changes resulting in floods and famine.
The scientists rushed to the conclusion, over the next few years, that the depletion of the ozone layer was due to chemicals known as chlorofluorocarbons, or CFCs, which are used in such things as aerosols, hamburger packaging and refrigerators. Most scientists agree that a reduction of 85 per cent in CFC emissions is needed immediately – just to stabilise conditions.
In Montreal, however, the top brains from twenty-four countries decided on a reduction of only 50 per cent, and then not until 1998. Moreover, they were talking only about consumption of CFCs. In fact, they’ve actually agreed to let the big companies increase their production of CFCs.
Negative consequences of ozone depletion
Adapted from: http://www.bcairquality.ca/101/ozone-depletion-impacts.html
Stratospheric ozone filters out most of the sun’s potentially harmful shortwave ultraviolet (UV) radiation. If this ozone becomes depleted, then more UV rays will reach the earth. Exposure to higher amounts of UV radiation could have serious impacts on human beings, animals and plants, such as the following:
Harm to human health

- More skin cancers, sunburns and premature aging of the skin.
- More cataracts, blindness and other eye diseases: UV radiation can damage several parts of the eye, including the lens, cornea, retina and conjunctiva.
- Cataracts (a clouding of the lens) are the major cause of blindness in the world. A sustained 10% thinning of the ozone layer is expected to result in almost two million new cases of cataracts per year, globally (Environment Canada, 1993).
- Weakening of the human immune system (immunosuppression). Early findings suggest that too much UV radiation can suppress the human immune system, which may play a role in the development of skin cancer.
Adverse impacts on agriculture, forestry and natural ecosystems

- Several of the world’s major crop species are particularly vulnerable to increased UV, resulting in reduced growth, photosynthesis and flowering. These species include wheat, rice, barley, oats, corn, soybeans, peas, tomatoes, cucumbers, cauliflower, broccoli and carrots.
- The effect of ozone depletion on the Canadian agricultural sector could be significant.
- Only a few commercially important trees have been tested for UV (UV-B) sensitivity, but early results suggest that plant growth, especially in seedlings, is harmed by more intense UV radiation.
Damage to marine life

- In particular, plankton (tiny organisms in the surface layer of oceans) are threatened by increased UV radiation. Plankton are the first vital step in aquatic food chains.
- Decreases in plankton could disrupt the fresh and saltwater food chains, and lead to a species shift in Canadian waters.
- Loss of biodiversity in our oceans, rivers and lakes could reduce fish yields for commercial and sport fisheries.
Damages to animals

- In domestic animals, UV overexposure may cause eye and skin cancers. Species of marine animals in their developmental stage (e.g. young fish, shrimp larvae and crab larvae) have been threatened in recent years by the increased UV radiation under the Antarctic ozone hole.
Materials

- Wood, plastic, rubber, fabrics and many construction materials are degraded by UV radiation.
- The economic impact of replacing and/or protecting materials could be significant.

