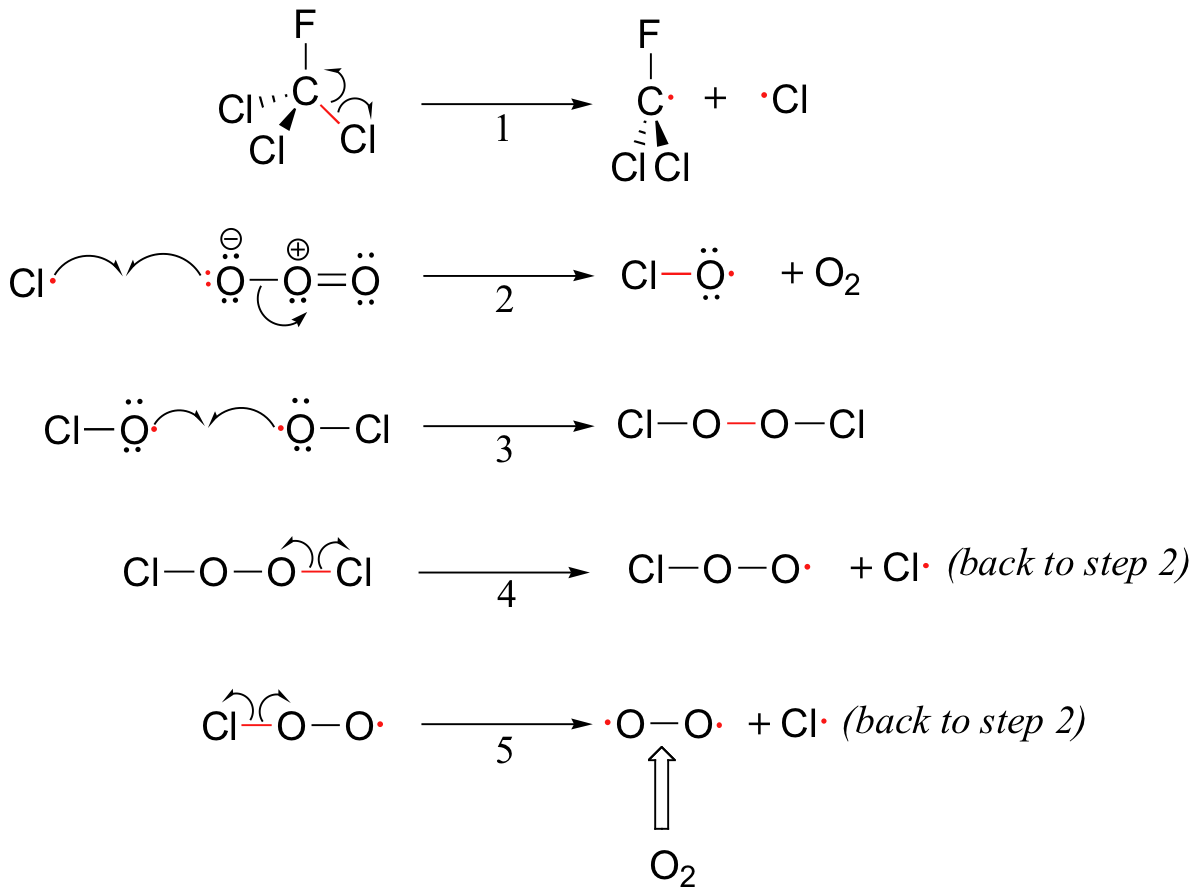
 Figure 1. CFC Radical Mechanism (Tim Soderberg, 2014).
Figure 1. CFC Radical Mechanism (Tim Soderberg, 2014).
Initiation step
Equation (1) Under the presence of UV light/heat, CFC will undergo homolytic fission to produce ·CCl2F and Cl· radical.
Propagation step
Equation (2) The chlorine radical (Cl·) will then react with Ozone to form ClO· radical and oxygen gas.
Termination step
Equation (3) 2 ClO· radicals reacted together to form the product Cl2O2.
Other steps
Equation (4) Cl2O2 (chlorine peroxide) will undergo homolytic fission under the presence of UV light/heat to form Cl· and ClO2· radical.
Equation (5)
ClO2· radical will undergo homolytic fission under the presence of UV light/ heat to produce O2 and Cl· radical.
In this case, Cl· is a catalyst to the above mechanism.
