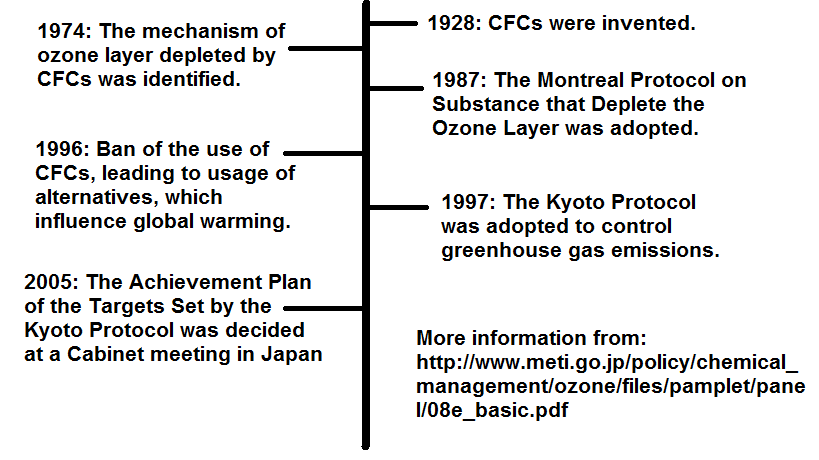
Figure 1 : History of CFCs
Ever since 1974 when the scientists published their first scientific hypotheses that the chemicals we produced could harm the stratosphere ozone layer, there is a global concern on ozone depletion/hole. In 1977, the United Nations Environment Programme (UNEP) concluded a World Plan of Action on the Ozone Layer. And in 1981, UNEP’s Governing Council authorized UNEP to draft a global framework convention on stratospheric ozone protection. This lead to the Vienna Convention concluded in 1985.
The Vienna Convention for the protection of the ozone layer
The Vienna Convention for the Protection of the Ozone Layer was adopted in 1985 and entered into force on 22 September 1988. In 2009, the Vienna Convention became the first Convention of any kind to achieve universal ratification. The objectives of the Convention were for Parties to promote cooperation by means of systematic observations, research and information exchange of the effects on human activities on the ozone layer and to adopt legislative or administrative measures against activities likely to have adverse effects on the Ozone layer.
197 countries were involved in the Vienna Convention.
The Montreal Protocol on Substances that deplete the ozone layer
During the Vienna Convention, some countries discussed a possible protocol to provide specific targets for the certain chemicals, but no censures was reached. A working group under the UNEP begins negotiations on the protocol and was agreed on 16 September 1987, entered into force on 1 January 1989. The Montreal Protocol on Substances that deplete the ozone layer was designed to reduce the production and consumption of ozone depleting substances, in order to reduce their abundance into the atmosphere, and thereby protecting the Earth’s fragile ozone layer.
Replacement of CFCs
Hydrochlorofluorocarbon (HCFC) is a haloalkane containing hydrogen, carbon, fluorine and chlorine atoms. HCFCs were the first substances used for replacement for CFCs. The carbon-hydrogen (C-H) bonds are susceptible to attack by radicals and atoms in the troposphere, and are therefore decomposed there to a significant extent. However, they are still able to diffuse into the stratosphere and cause sufficient ozone depletion.

Figure 2: Lewis Structure of Hydrochloroflurocarbon (HCFC)
Taken from: https://fi.wikipedia.org/wiki/HCFC-yhdisteet
Hydrofluorocarbon (HFC) is another haloalkane containing hydrogen, carbon and fluorine atoms. HFCs are now commonly used to replace CFCs. HFCs have similar traits as HCFCs, the carbon-hydrogen (C-H) bonds could decompose to a significant extent at the troposphere. The difference between HCFCs, CFCs and HFCs is HFCs contain no carbon-chlorine (C-Cl) bonds that will react to form Cl. radical in the stratosphere. Thus, the ozone-destroying capacity of HCFs is zero. The most widely used HFCs is 1,1,1,2-tetrafluoroethane, which is used for refrigerant and air conditioning. It is more expensive and less efficient than CFCs it replaces.
 Figure 3: Structure of 1,1,1,2-tetrafluoroethane
Figure 3: Structure of 1,1,1,2-tetrafluoroethane
Hydrocarbon is an organic compound containing only hydrogen and carbon atoms. Hydrocarbons have replaced CFCs as propellants in spray cans.
References
Edith Brown Weiss, n.d. Vienna Convention for the Protection of the Ozone Layer, 1985, and Montreal Protocol on Substances that Deplete the Ozone Layer, 1987. Taken from: http://legal.un.org/avl/ha/vcpol/vcpol.html
Ozone Secretariat, n.d. The Vienna Convention for the Protection of Ozone Layer. Taken from: http://ozone.unep.org/en/treaties-and-decisions/vienna-convention-protection-ozone-layer
United Nation Treaty Collection, n.d. Vienna Convention for the Protection of Ozone Layer. Taken from: https://treaties.un.org/pages/ViewDetails.aspx?src=TREATY&mtdsg_no=XXVII-2&chapter=27&clang=_en
Ozone Secretariat, n.d. The Montreal Protocol on Substances that Deplete the Ozone Layer. Taken from: http://ozone.unep.org/en/treaties-and-decisions/montreal-protocol-substances-deplete-ozone-layer
Matt Schiller, n.d. Alternatives to CFCs. Taken from: http://www.easychem.com.au/monitoring-and-management/the-atmosphere/alternatives-to-cfcs