Ozone is a pungent gas that is made up of 3 oxygen atoms. It is an allotrope of oxygen that is much less stable then the diatomic allotrope O2. It can be found in small concentrations throughout the Earth’s atmosphere (stratosphere).

Figure 1. Earth Ozone layer (NC State University, 2013)
For the basic bonding, ozone has a strong intramolecular covalent bonding between the atoms and a weak intermolecular London dispersion force between the molecules. Low energy is needed to overcome these weak intermolecular forces of attraction and thus, ozone exists in gaseous state in standard room temperature and pressure.
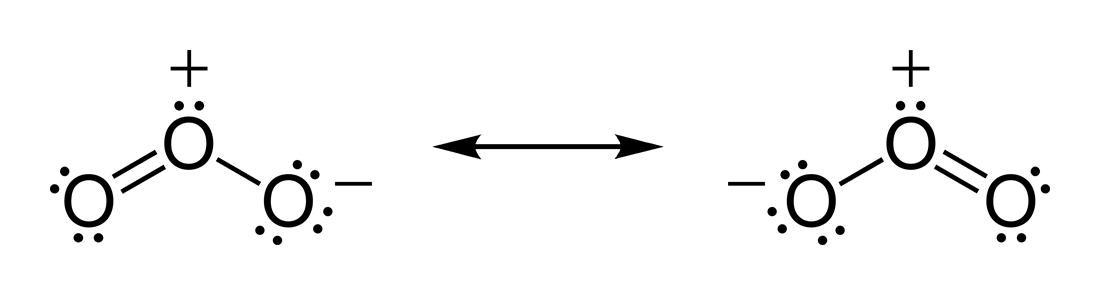 Figure 2. Ozone resonance structure (Wikipedia, 2017)
Figure 2. Ozone resonance structure (Wikipedia, 2017)
As shown in the diagram above, since ozone has multiple bonds, a single Lewis structure does not adequately represent the true structure of a molecule. Resonance forms is used in this case. In chemistry, resonance is a way of describing the delocalised electrons within certain molecules or polyatomic ions where bonding cannot be expressed by one single Lewis structure. Ozone can be existed either the left or right structure.
